
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 22E
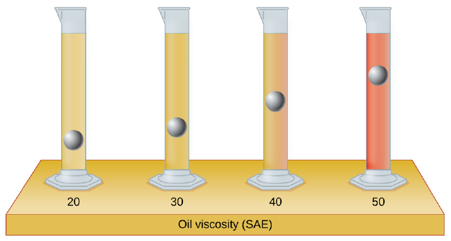
The test tubes shown here contain equal amounts of the specified motor oils. Identical metal spheres were dropped at the same time into each of the tubes, and a brief moment later, the spheres had fallen to the heights indicated in the illustration.
Rank the motor oils in order of increasing viscosity, and explain your reasoning:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Can someone help me whats the issue?
a. The change in the Gibbs energy of a certain constant pressure process is found to fit the expression:
AG-85.1 J mol −1 +36.5 J mol ¹K-1 × T
A. Calculate the value of AS for the process.
B. Next, use the Gibbs-Helmholtz equation:
(a(AG/T))
ΔΗ
-
T2
to calculate the value of AH for the process.
None
Chapter 10 Solutions
Chemistry by OpenStax (2015-05-04)
Ch. 10 - In terms of their bulk properties, how do liquids...Ch. 10 - In terms of the kinetic molecular theory, in what...Ch. 10 - In terms of the kinetic molecular theory, in what...Ch. 10 - Explain why liquids assume the shape of any...Ch. 10 - What is the evidence that all neutral atoms and...Ch. 10 - Open the PhET States of Matter Simulation...Ch. 10 - Define the following and give an example of each:...Ch. 10 - The types of intermolecular forces in a substance...Ch. 10 - Why do the boiling points of the noble gases...Ch. 10 - Neon and HF have approximately the same molecular...
Ch. 10 - Arrange each of the following sets of compounds in...Ch. 10 - The molecular mass of butanol, C4H9OH, is 74.14;...Ch. 10 - On the basis of intermolecular attractions,...Ch. 10 - On the basis of dipole moments and/or hydrogen...Ch. 10 - The melting point of H2O(s) is O C. Would you...Ch. 10 - Silane SiH4, phosphine (PH3), and hydrogen sulfide...Ch. 10 - Explain why a hydrogen bond between two water...Ch. 10 - Under certain conditions, molecules of acetic...Ch. 10 - Proteins are chains of amino acids that can form...Ch. 10 - The density of liquid NH3 is 0.64 g/mL; the...Ch. 10 - Identify the intermolecular forces present in the...Ch. 10 - The test tubes shown here contain equal amounts of...Ch. 10 - Although steel is denser than water, a steel...Ch. 10 - The surface tension and viscosity values for...Ch. 10 - You may have heard someone use the figure of...Ch. 10 - It is often recommended that you let your car...Ch. 10 - The surface tension and viscosity of water at...Ch. 10 - At 25 C, how high will water rise in a glass...Ch. 10 - Water rises in a glass capillary tube to a height...Ch. 10 - Heat is added to boiling water. Explain why the...Ch. 10 - Heat is added to ice at 0 C. Explain why the...Ch. 10 - What feature characterizes the dynamic equilibrium...Ch. 10 - Identify two common observations indicating some...Ch. 10 - Identify two common observations indicating some...Ch. 10 - What is the relationship between the...Ch. 10 - What is the relationship between the...Ch. 10 - Why does spilled gasoline evaporate more rapidly...Ch. 10 - Carbon tetrachloride, CCl4, was once used as a dry...Ch. 10 - When is the boiling point of a liquid equal to its...Ch. 10 - How does the boiling of a liquid differ from its...Ch. 10 - Use the information in Figure 10.24 to estimate...Ch. 10 - A syringe at a temperature of 20 C is filled with...Ch. 10 - Explain the following observations: (a) It takes...Ch. 10 - The enthalpy of vaporization of water is larger...Ch. 10 - Explain why the molar enthalpies of vaporization...Ch. 10 - Explain why the enthalpies of vaporization of the...Ch. 10 - The enthalpy of vaporization of CO2(l) is 9.8...Ch. 10 - The hydrogen fluoride molecule, HF, is more polar...Ch. 10 - Ethyl chloride (boiling point, 13 C) is used as a...Ch. 10 - Which contains the compounds listed correctly in...Ch. 10 - How much heat is required to convert 422 g of...Ch. 10 - Evaporation of sweat requires energy and thus take...Ch. 10 - Titanium tetrachloride, TiCl4, has a melting point...Ch. 10 - From the phase diagram for water (Figure 10.31),...Ch. 10 - What phase changes will take place when water is...Ch. 10 - Pressure cookers allow food to cook faster because...Ch. 10 - From the phase diagram for carbon dioxide in...Ch. 10 - Determine the phase changes that carbon dioxide...Ch. 10 - Consider a cylinder containing a mixture of liquid...Ch. 10 - Dry ice, CO2(s) , does not melt at atmospheric...Ch. 10 - If a severe storm results in the loss of...Ch. 10 - Is it possible to liquefy nitrogen at room...Ch. 10 - Elemental carbon has one gas phase, one liquid...Ch. 10 - What types of liquids typically form amorphous...Ch. 10 - At very low temperatures oxygen, O2, freezes and...Ch. 10 - As it cools, olive oil slowly solidifies and forms...Ch. 10 - Explain why ice, which is a crystalline solid, has...Ch. 10 - Identify the type of crystalline solid (metallic,...Ch. 10 - Identify the type of crystalline solid (metallic,...Ch. 10 - Classify each substance in the table as either a...Ch. 10 - Classify each substance in the table as either a...Ch. 10 - Identify the following substances as ionic,...Ch. 10 - Substance A is shiny, conducts electricity well,...Ch. 10 - Substance B is hard, does not conduct electricity,...Ch. 10 - Describe the crystal structure of iron, which...Ch. 10 - Describe the crystal structure of Pt, which...Ch. 10 - What is the coordination number of a chromium atom...Ch. 10 - What is the coordination number of an aluminum...Ch. 10 - Cobalt metal crystallizes in a hexagonal closest...Ch. 10 - Nickel metal crystallizes in a cubic closest...Ch. 10 - Tungsten crystallizes in a body-centered cubic...Ch. 10 - Platinum (atomic radius =1.38) crystallizes in a...Ch. 10 - Barium crystallizes in a body-centered cubic unit...Ch. 10 - Aluminum (atomic radius = 1.43 ) crystallizes in a...Ch. 10 - The density of aluminum is 2.7 g/cm3; that of...Ch. 10 - The free space in a metal may be found by...Ch. 10 - Cadmium sulfide, sometimes used as a yellow...Ch. 10 - A compound of cadmium, tin, and phosphorus is used...Ch. 10 - What is the formula of the magnetic oxide of...Ch. 10 - A compound containing zinc, aluminum, and sulfur...Ch. 10 - A compound of thallium and iodine crystallizes in...Ch. 10 - Which of the following elements reacts with sulfur...Ch. 10 - What is the percent by mass of titanium in rutile,...Ch. 10 - Explain why the chemically similar alkali metal...Ch. 10 - As minerals were formed from the molten magma,...Ch. 10 - Rubidium iodide crystallizes with a cubic unit...Ch. 10 - One of the various manganese oxides crystallizes...Ch. 10 - NaH crystallizes with the same ciystal structure...Ch. 10 - Thallium(I) iodide crystallizes with the same...Ch. 10 - A cubic unit cell contains manganese ions at the...Ch. 10 - What is the spacing between crystal planes that...Ch. 10 - A diffracrometer using X-rays with a wavelength of...Ch. 10 - A metal with spacing between planes equal to...Ch. 10 - Gold crystallizes in a face-centered cubic unit...Ch. 10 - When an electron in an excited molybdenum atom...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Endospore formation is called (a) _____. It is initiated by (b) _____. Formation of a new cell from an endospor...
Microbiology: An Introduction
49. For the reaction shown, calculate the theoretical yield of product in moles for each of the initial quantit...
Introductory Chemistry (6th Edition)
If isomer A is heated to about 100 C, a mixture of isomers A and B is formed. Explain why there is no trace of ...
Organic Chemistry (8th Edition)
Which type of cartilage is most plentiful in the adult body?
Anatomy & Physiology (6th Edition)
Some organizations are starting to envision a sustainable societyone in which each generation inherits sufficie...
Campbell Essential Biology (7th Edition)
In what way do the membranes of a eukaryotic cell vary? A. Phospholipids are found only in certain membranes. B...
Campbell Biology in Focus (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ASP please....arrow_forwardNonearrow_forwardConsider the structure of 1-bromo-2-fluoroethane. Part 1 of 2 Draw the Newman projection for the anti conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. ✡ ぬ Part 2 of 2 H H F Br H H ☑ Draw the Newman projection for the gauche conformation of 1-bromo-2-fluoroethane, viewed down the C1-C2 bond. H F Br H Harrow_forward
- Please help me answer this question. I don't understand how or where the different reagents will attach and it's mostly due to the wedge bond because I haven't seen a problem like this before. Please provide a detailed explanation and a drawing showing how it can happen and what the final product will look like.arrow_forwardWhich of the following compounds is the most acidic in the gas phase? Group of answer choices H2O SiH4 HBr H2Sarrow_forwardWhich of the following is the most acidic transition metal cation? Group of answer choices Fe3+ Sc3+ Mn4+ Zn2+arrow_forward
- Based on the thermodynamics of acetic acid dissociation discussed in Lecture 2-5, what can you conclude about the standard enthalpy change (ΔHo) of acid dissociation for HCl? Group of answer choices You cannot arrive at any of the other three conclusions It is a positive value It is more negative than −0.4 kJ/mol It equals −0.4 kJ/molarrow_forwardPLEASE HELP URGENT!arrow_forwardDraw the skeletal structure corresponding to the following IUPAC name: 7-isopropyl-3-methyldecanearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
