
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 41E
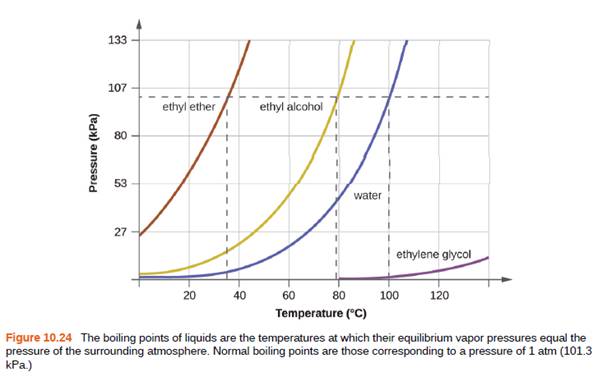
Use the information in Figure 10.24 to estimate the boiling point of water in Denver when the atmospheric pressure is 83.3 kPa.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating
the reactants?
O
? A
. If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like.
. If your answer is no, check the box under the drawing area instead.
Explanation
Check
Click and drag to start drawing a structure.
ㅇ
80
F5
F6
A
2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cente
FIG
In methyl orange preparation, if the reaction started with 0.5 mole of sulfanilic acid to form the diazonium salt of this compound and then it converted to methyl orange [0.2 mole]. If the efficiency of the second step was 50%, Calculate: A. Equation(s) of Methyl Orange synthesis: Diazotization and coupling reactions. B. How much diazonium salt was formed in this reaction? C. The efficiency percentage of the diazotization reaction D. Efficiency percentage of the whole reaction.
Hand written equations please
Chapter 10 Solutions
Chemistry by OpenStax (2015-05-04)
Ch. 10 - In terms of their bulk properties, how do liquids...Ch. 10 - In terms of the kinetic molecular theory, in what...Ch. 10 - In terms of the kinetic molecular theory, in what...Ch. 10 - Explain why liquids assume the shape of any...Ch. 10 - What is the evidence that all neutral atoms and...Ch. 10 - Open the PhET States of Matter Simulation...Ch. 10 - Define the following and give an example of each:...Ch. 10 - The types of intermolecular forces in a substance...Ch. 10 - Why do the boiling points of the noble gases...Ch. 10 - Neon and HF have approximately the same molecular...
Ch. 10 - Arrange each of the following sets of compounds in...Ch. 10 - The molecular mass of butanol, C4H9OH, is 74.14;...Ch. 10 - On the basis of intermolecular attractions,...Ch. 10 - On the basis of dipole moments and/or hydrogen...Ch. 10 - The melting point of H2O(s) is O C. Would you...Ch. 10 - Silane SiH4, phosphine (PH3), and hydrogen sulfide...Ch. 10 - Explain why a hydrogen bond between two water...Ch. 10 - Under certain conditions, molecules of acetic...Ch. 10 - Proteins are chains of amino acids that can form...Ch. 10 - The density of liquid NH3 is 0.64 g/mL; the...Ch. 10 - Identify the intermolecular forces present in the...Ch. 10 - The test tubes shown here contain equal amounts of...Ch. 10 - Although steel is denser than water, a steel...Ch. 10 - The surface tension and viscosity values for...Ch. 10 - You may have heard someone use the figure of...Ch. 10 - It is often recommended that you let your car...Ch. 10 - The surface tension and viscosity of water at...Ch. 10 - At 25 C, how high will water rise in a glass...Ch. 10 - Water rises in a glass capillary tube to a height...Ch. 10 - Heat is added to boiling water. Explain why the...Ch. 10 - Heat is added to ice at 0 C. Explain why the...Ch. 10 - What feature characterizes the dynamic equilibrium...Ch. 10 - Identify two common observations indicating some...Ch. 10 - Identify two common observations indicating some...Ch. 10 - What is the relationship between the...Ch. 10 - What is the relationship between the...Ch. 10 - Why does spilled gasoline evaporate more rapidly...Ch. 10 - Carbon tetrachloride, CCl4, was once used as a dry...Ch. 10 - When is the boiling point of a liquid equal to its...Ch. 10 - How does the boiling of a liquid differ from its...Ch. 10 - Use the information in Figure 10.24 to estimate...Ch. 10 - A syringe at a temperature of 20 C is filled with...Ch. 10 - Explain the following observations: (a) It takes...Ch. 10 - The enthalpy of vaporization of water is larger...Ch. 10 - Explain why the molar enthalpies of vaporization...Ch. 10 - Explain why the enthalpies of vaporization of the...Ch. 10 - The enthalpy of vaporization of CO2(l) is 9.8...Ch. 10 - The hydrogen fluoride molecule, HF, is more polar...Ch. 10 - Ethyl chloride (boiling point, 13 C) is used as a...Ch. 10 - Which contains the compounds listed correctly in...Ch. 10 - How much heat is required to convert 422 g of...Ch. 10 - Evaporation of sweat requires energy and thus take...Ch. 10 - Titanium tetrachloride, TiCl4, has a melting point...Ch. 10 - From the phase diagram for water (Figure 10.31),...Ch. 10 - What phase changes will take place when water is...Ch. 10 - Pressure cookers allow food to cook faster because...Ch. 10 - From the phase diagram for carbon dioxide in...Ch. 10 - Determine the phase changes that carbon dioxide...Ch. 10 - Consider a cylinder containing a mixture of liquid...Ch. 10 - Dry ice, CO2(s) , does not melt at atmospheric...Ch. 10 - If a severe storm results in the loss of...Ch. 10 - Is it possible to liquefy nitrogen at room...Ch. 10 - Elemental carbon has one gas phase, one liquid...Ch. 10 - What types of liquids typically form amorphous...Ch. 10 - At very low temperatures oxygen, O2, freezes and...Ch. 10 - As it cools, olive oil slowly solidifies and forms...Ch. 10 - Explain why ice, which is a crystalline solid, has...Ch. 10 - Identify the type of crystalline solid (metallic,...Ch. 10 - Identify the type of crystalline solid (metallic,...Ch. 10 - Classify each substance in the table as either a...Ch. 10 - Classify each substance in the table as either a...Ch. 10 - Identify the following substances as ionic,...Ch. 10 - Substance A is shiny, conducts electricity well,...Ch. 10 - Substance B is hard, does not conduct electricity,...Ch. 10 - Describe the crystal structure of iron, which...Ch. 10 - Describe the crystal structure of Pt, which...Ch. 10 - What is the coordination number of a chromium atom...Ch. 10 - What is the coordination number of an aluminum...Ch. 10 - Cobalt metal crystallizes in a hexagonal closest...Ch. 10 - Nickel metal crystallizes in a cubic closest...Ch. 10 - Tungsten crystallizes in a body-centered cubic...Ch. 10 - Platinum (atomic radius =1.38) crystallizes in a...Ch. 10 - Barium crystallizes in a body-centered cubic unit...Ch. 10 - Aluminum (atomic radius = 1.43 ) crystallizes in a...Ch. 10 - The density of aluminum is 2.7 g/cm3; that of...Ch. 10 - The free space in a metal may be found by...Ch. 10 - Cadmium sulfide, sometimes used as a yellow...Ch. 10 - A compound of cadmium, tin, and phosphorus is used...Ch. 10 - What is the formula of the magnetic oxide of...Ch. 10 - A compound containing zinc, aluminum, and sulfur...Ch. 10 - A compound of thallium and iodine crystallizes in...Ch. 10 - Which of the following elements reacts with sulfur...Ch. 10 - What is the percent by mass of titanium in rutile,...Ch. 10 - Explain why the chemically similar alkali metal...Ch. 10 - As minerals were formed from the molten magma,...Ch. 10 - Rubidium iodide crystallizes with a cubic unit...Ch. 10 - One of the various manganese oxides crystallizes...Ch. 10 - NaH crystallizes with the same ciystal structure...Ch. 10 - Thallium(I) iodide crystallizes with the same...Ch. 10 - A cubic unit cell contains manganese ions at the...Ch. 10 - What is the spacing between crystal planes that...Ch. 10 - A diffracrometer using X-rays with a wavelength of...Ch. 10 - A metal with spacing between planes equal to...Ch. 10 - Gold crystallizes in a face-centered cubic unit...Ch. 10 - When an electron in an excited molybdenum atom...
Additional Science Textbook Solutions
Find more solutions based on key concepts
2 Of the uterus, small intestine, spinal cord, and heart, which is/are in the dorsal body cavity?
Anatomy & Physiology (6th Edition)
2. List the subdivisions of the thoracic and abdominopelvic cavities.
Human Anatomy & Physiology (2nd Edition)
1. ___ Mitosis 2. ___ Meiosis 3. __ Homologous chromosomes 4. __ Crossing over 5. __ Cytokinesis A. Cytoplasmic...
Microbiology with Diseases by Body System (5th Edition)
Fibrous connective tissue consists of ground substance and fibers that provide strength, support, and flexibili...
Human Biology: Concepts and Current Issues (8th Edition)
MAKE CONNECTIONS Which chemical group is most likely to be responsible for an organic molecule behaving as a ba...
Campbell Biology (11th Edition)
As genetic testing becomes widespread, medical records will contain the results of such testing. Who should hav...
Concepts of Genetics (12th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hand written equations pleasearrow_forward> each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X Ś CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) © 2025 McGraw Hill LLC. All Rights Farrow_forwardNMR spectrum of ethyl acetate has signals whose chemical shifts are indicated below. Which hydrogen or set of hydrogens corresponds to the signal at 4.1 ppm? Select the single best answer. The H O HỌC—C—0—CH, CH, 2 A ethyl acetate H NMR: 1.3 ppm, 2.0 ppm, 4.1 ppm Check OA B OC ch B C Save For Later Submit Ass © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |arrow_forward
- How many signals do you expect in the H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red Note for advanced students: In this question, any multiplet is counted as one signal. 1 Number of signals in the 'H NMR spectrum. For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. Check For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. O ✓ No additional Hs to color in top molecule ง No additional Hs to color in bottom…arrow_forwardin the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstantarrow_forwardtrue or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forward
- in the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forwardtrue or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forward
- I2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forwardtrue or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY