
An Introduction to Physical Science
14th Edition
ISBN: 9781305079120
Author: James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher: Brooks Cole
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 1VC
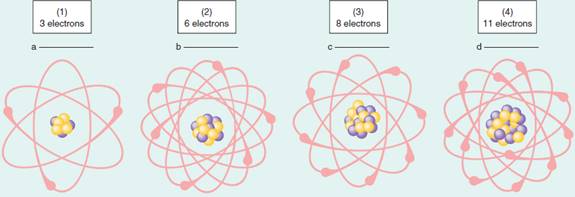
Visualize the connections and give the nuclear symbol
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
An aluminum rod and a copper rod have the same length of 100cm at 5C. At what temperatures would one of the rods be 0.5 mm longer than the other? Which rod is longer at such temperature?
ROTATIONAL DYNAMICS
Question 01
A solid circular cylinder and a solid spherical ball of the same mass and radius are rolling
together down the same inclined. Calculate the ratio of their kinetic energy. Assume pure
rolling motion Question 02
A sphere and cylinder of the same mass and radius start from ret at the same point and more
down the same plane inclined at 30° to the horizontal
Which body gets the bottom first and what is its acceleration
b) What angle of inclination of the plane is needed to give the slower body the same
acceleration
Question 03
i)
Define the angular velocity of a rotating body and give its SI unit
A car wheel has its angular velocity changing from 2rads to 30 rads
seconds. If the radius of the wheel is 400mm. calculate
ii)
The angular acceleration
iii)
The tangential linear acceleration of a point on the rim of the wheel
Question 04
in 20
Question B3
Consider the following FLRW spacetime:
t2
ds² = -dt² +
(dx²
+ dy²+ dz²),
t2
where t is a constant.
a)
State whether this universe is spatially open, closed or flat.
[2 marks]
b) Determine the Hubble factor H(t), and represent it in a (roughly drawn) plot as a function
of time t, starting at t = 0.
[3 marks]
c) Taking galaxy A to be located at (x, y, z) = (0,0,0), determine the proper distance to galaxy
B located at (x, y, z) = (L, 0, 0). Determine the recessional velocity of galaxy B with respect
to galaxy A.
d) The Friedmann equations are
2
k
8πG
а
4πG
+
a²
(p+3p).
3
a
3
[5 marks]
Use these equations to determine the energy density p(t) and the pressure p(t) for the
FLRW spacetime specified at the top of the page.
[5 marks]
e) Given the result of question B3.d, state whether the FLRW universe in question is (i)
radiation-dominated, (ii) matter-dominated, (iii) cosmological-constant-dominated, or (iv)
none of the previous. Justify your answer.
f)
[5 marks]
A conformally…
Chapter 10 Solutions
An Introduction to Physical Science
Ch. 10.1 - What original elements did Aristotle think...Ch. 10.1 - Prob. 2PQCh. 10.2 - Prob. 1PQCh. 10.2 - Prob. 2PQCh. 10.2 - Prob. 10.1CECh. 10.3 - Prob. 1PQCh. 10.3 - Prob. 2PQCh. 10.3 - Prob. 10.2CECh. 10.3 - Prob. 10.3CECh. 10.3 - Prob. 10.4CE
Ch. 10.3 - Prob. 10.5CECh. 10.3 - Prob. 10.6CECh. 10.4 - What quantities are conserved in nuclear...Ch. 10.4 - Prob. 2PQCh. 10.4 - Prob. 10.7CECh. 10.5 - Prob. 1PQCh. 10.5 - Prob. 2PQCh. 10.5 - Prob. 10.8CECh. 10.6 - Where does nuclear fusion occur naturally?Ch. 10.6 - Prob. 2PQCh. 10.6 - Prob. 10.9CECh. 10.7 - Prob. 1PQCh. 10.7 - Prob. 2PQCh. 10.8 - Prob. 1PQCh. 10.8 - Prob. 2PQCh. 10 - Prob. AMCh. 10 - Prob. BMCh. 10 - Prob. CMCh. 10 - Prob. DMCh. 10 - Prob. EMCh. 10 - Prob. FMCh. 10 - Prob. GMCh. 10 - Prob. HMCh. 10 - Prob. IMCh. 10 - Prob. JMCh. 10 - Prob. KMCh. 10 - Prob. LMCh. 10 - Prob. MMCh. 10 - Prob. NMCh. 10 - Prob. OMCh. 10 - Prob. PMCh. 10 - Prob. QMCh. 10 - Prob. RMCh. 10 - Prob. SMCh. 10 - Prob. TMCh. 10 - Prob. UMCh. 10 - Prob. VMCh. 10 - Prob. WMCh. 10 - Prob. XMCh. 10 - Prob. YMCh. 10 - Prob. ZMCh. 10 - Prob. 1MCCh. 10 - Prob. 2MCCh. 10 - Prob. 3MCCh. 10 - Prob. 4MCCh. 10 - Which radioactive decay mode does not result in a...Ch. 10 - Prob. 6MCCh. 10 - Prob. 7MCCh. 10 - Prob. 8MCCh. 10 - How many half-lives would it take for a sample of...Ch. 10 - Which of the following is not conserved in all...Ch. 10 - Prob. 11MCCh. 10 - Prob. 12MCCh. 10 - Prob. 13MCCh. 10 - Which unit is most closely associated with the...Ch. 10 - Prob. 15MCCh. 10 - Prob. 1FIBCh. 10 - Prob. 2FIBCh. 10 - The collective name for neutrons and protons in a...Ch. 10 - Prob. 4FIBCh. 10 - No stable nuclides exist that have Z greater than...Ch. 10 - Prob. 6FIBCh. 10 - The amount of a radioactive isotope will have...Ch. 10 - Prob. 8FIBCh. 10 - Prob. 9FIBCh. 10 - For an atomic bomb to explode, a(n) ____ mass is...Ch. 10 - In discussions of nuclear fusion reactions, the...Ch. 10 - Prob. 12FIBCh. 10 - Prob. 1SACh. 10 - Prob. 2SACh. 10 - Prob. 3SACh. 10 - Prob. 4SACh. 10 - Prob. 5SACh. 10 - What evidence is there to support the idea that...Ch. 10 - Prob. 7SACh. 10 - Prob. 8SACh. 10 - Prob. 9SACh. 10 - Prob. 10SACh. 10 - Prob. 11SACh. 10 - Prob. 12SACh. 10 - Prob. 13SACh. 10 - Prob. 14SACh. 10 - Prob. 15SACh. 10 - Prob. 16SACh. 10 - Prob. 17SACh. 10 - After three half-lives have gone by, what fraction...Ch. 10 - Prob. 19SACh. 10 - Prob. 20SACh. 10 - Prob. 21SACh. 10 - Prob. 22SACh. 10 - Prob. 23SACh. 10 - Prob. 24SACh. 10 - Prob. 25SACh. 10 - Prob. 26SACh. 10 - Prob. 27SACh. 10 - Prob. 28SACh. 10 - Prob. 29SACh. 10 - Prob. 30SACh. 10 - Prob. 31SACh. 10 - Prob. 32SACh. 10 - Prob. 33SACh. 10 - Prob. 34SACh. 10 - Prob. 35SACh. 10 - Prob. 36SACh. 10 - Prob. 37SACh. 10 - Prob. 38SACh. 10 - Prob. 39SACh. 10 - Prob. 40SACh. 10 - Prob. 41SACh. 10 - Prob. 42SACh. 10 - Visualize the connections and give the nuclear...Ch. 10 - Prob. 1AYKCh. 10 - The technique of carbon-14 dating relies on the...Ch. 10 - Prob. 3AYKCh. 10 - Prob. 4AYKCh. 10 - Prob. 5AYKCh. 10 - Fill in the nine gaps in this table.Ch. 10 - Fill in the nine gaps in this table.Ch. 10 - Prob. 3ECh. 10 - Prob. 4ECh. 10 - Prob. 5ECh. 10 - Prob. 6ECh. 10 - Prob. 7ECh. 10 - Actinium-225 (89225Ac) undergoes alpha decay. (a)...Ch. 10 - Prob. 9ECh. 10 - Prob. 10ECh. 10 - Prob. 11ECh. 10 - Prob. 12ECh. 10 - Prob. 13ECh. 10 - Prob. 14ECh. 10 - Prob. 15ECh. 10 - Prob. 16ECh. 10 - Prob. 17ECh. 10 - What is the half-life of thallium-206 if the...Ch. 10 - Use the graph in Fig. 10.24 to find the half-life...Ch. 10 - Prob. 20ECh. 10 - Prob. 21ECh. 10 - Prob. 22ECh. 10 - Prob. 23ECh. 10 - Prob. 24ECh. 10 - Prob. 25ECh. 10 - Prob. 26ECh. 10 - Prob. 27ECh. 10 - Prob. 28E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- SECTION B Answer ONLY TWO questions in Section B [Expect to use one single-sided A4 page for each Section-B sub question.] Question B1 Consider the line element where w is a constant. ds²=-dt²+e2wt dx², a) Determine the components of the metric and of the inverse metric. [2 marks] b) Determine the Christoffel symbols. [See the Appendix of this document.] [10 marks] c) Write down the geodesic equations. [5 marks] d) Show that e2wt it is a constant of geodesic motion. [4 marks] e) Solve the geodesic equations for null geodesics. [4 marks]arrow_forwardPage 2 SECTION A Answer ALL questions in Section A [Expect to use one single-sided A4 page for each Section-A sub question.] Question A1 SPA6308 (2024) Consider Minkowski spacetime in Cartesian coordinates th = (t, x, y, z), such that ds² = dt² + dx² + dy² + dz². (a) Consider the vector with components V" = (1,-1,0,0). Determine V and V. V. (b) Consider now the coordinate system x' (u, v, y, z) such that u =t-x, v=t+x. [2 marks] Write down the line element, the metric, the Christoffel symbols and the Riemann curvature tensor in the new coordinates. [See the Appendix of this document.] [5 marks] (c) Determine V", that is, write the object in question A1.a in the coordinate system x'. Verify explicitly that V. V is invariant under the coordinate transformation. Question A2 [5 marks] Suppose that A, is a covector field, and consider the object Fv=AAμ. (a) Show explicitly that F is a tensor, that is, show that it transforms appropriately under a coordinate transformation. [5 marks] (b)…arrow_forwardHow does boiling point of water decreases as the altitude increases?arrow_forward
- How would partial obstruction of an air intake port of an air-entrainment mask effect FiO2 and flow?arrow_forward14 Z In figure, a closed surface with q=b= 0.4m/ C = 0.6m if the left edge of the closed surface at position X=a, if E is non-uniform and is given by € = (3 + 2x²) ŷ N/C, calculate the (3+2x²) net electric flux leaving the closed surface.arrow_forwardNo chatgpt pls will upvotearrow_forward
- suggest a reason ultrasound cleaning is better than cleaning by hand?arrow_forwardCheckpoint 4 The figure shows four orientations of an electric di- pole in an external electric field. Rank the orienta- tions according to (a) the magnitude of the torque on the dipole and (b) the potential energy of the di- pole, greatest first. (1) (2) E (4)arrow_forwardWhat is integrated science. What is fractional distillation What is simple distillationarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
Half life | Radioactivity | Physics | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=IDkNlU7zKYU;License: Standard YouTube License, CC-BY