
An Introduction to Physical Science
14th Edition
ISBN: 9781305079120
Author: James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher: Brooks Cole
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 19E
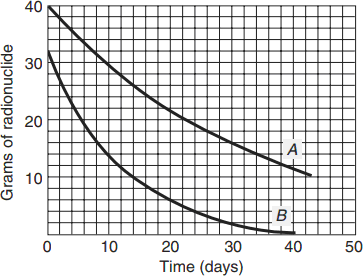
Use the graph in ● Fig. 10.24 to find the half-life of isotope A. All you need is a sound understanding of the definition of half-life.
Figure 10.24 See Exercise 19.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
You are standing a distance x = 1.75 m away from this mirror. The object you are looking at is y = 0.29 m from the mirror. The angle of incidence is θ = 30°. What is the exact distance from you to the image?
For each of the actions depicted below, a magnet and/or metal loop moves with velocity v→ (v→ is constant and has the same magnitude in all parts). Determine whether a current is induced in the metal loop. If so, indicate the direction of the current in the loop, either clockwise or counterclockwise when seen from the right of the loop. The axis of the magnet is lined up with the center of the loop. For the action depicted in (Figure 5), indicate the direction of the induced current in the loop (clockwise, counterclockwise or zero, when seen from the right of the loop). I know that the current is clockwise, I just dont understand why. Please fully explain why it's clockwise, Thank you
A planar double pendulum consists of two point masses \[m_1 = 1.00~\mathrm{kg}, \qquad m_2 = 1.00~\mathrm{kg}\]connected by massless, rigid rods of lengths \[L_1 = 1.00~\mathrm{m}, \qquad L_2 = 1.20~\mathrm{m}.\]The upper rod is hinged to a fixed pivot; gravity acts vertically downward with\[g = 9.81~\mathrm{m\,s^{-2}}.\]Define the generalized coordinates \(\theta_1,\theta_2\) as the angles each rod makes with thedownward vertical (positive anticlockwise, measured in radians unless stated otherwise).At \(t=0\) the system is released from rest with \[\theta_1(0)=120^{\circ}, \qquad\theta_2(0)=-10^{\circ}, \qquad\dot{\theta}_1(0)=\dot{\theta}_2(0)=0 .\]Using the exact nonlinear equations of motion (no small-angle or planar-pendulumapproximations) and assuming the rods never stretch or slip, determine the angle\(\theta_2\) at the instant\[t = 10.0~\mathrm{s}.\]Give the result in degrees, in the interval \((-180^{\circ},180^{\circ}]\).
Chapter 10 Solutions
An Introduction to Physical Science
Ch. 10.1 - What original elements did Aristotle think...Ch. 10.1 - Prob. 2PQCh. 10.2 - Prob. 1PQCh. 10.2 - Prob. 2PQCh. 10.2 - Prob. 10.1CECh. 10.3 - Prob. 1PQCh. 10.3 - Prob. 2PQCh. 10.3 - Prob. 10.2CECh. 10.3 - Prob. 10.3CECh. 10.3 - Prob. 10.4CE
Ch. 10.3 - Prob. 10.5CECh. 10.3 - Prob. 10.6CECh. 10.4 - What quantities are conserved in nuclear...Ch. 10.4 - Prob. 2PQCh. 10.4 - Prob. 10.7CECh. 10.5 - Prob. 1PQCh. 10.5 - Prob. 2PQCh. 10.5 - Prob. 10.8CECh. 10.6 - Where does nuclear fusion occur naturally?Ch. 10.6 - Prob. 2PQCh. 10.6 - Prob. 10.9CECh. 10.7 - Prob. 1PQCh. 10.7 - Prob. 2PQCh. 10.8 - Prob. 1PQCh. 10.8 - Prob. 2PQCh. 10 - Prob. AMCh. 10 - Prob. BMCh. 10 - Prob. CMCh. 10 - Prob. DMCh. 10 - Prob. EMCh. 10 - Prob. FMCh. 10 - Prob. GMCh. 10 - Prob. HMCh. 10 - Prob. IMCh. 10 - Prob. JMCh. 10 - Prob. KMCh. 10 - Prob. LMCh. 10 - Prob. MMCh. 10 - Prob. NMCh. 10 - Prob. OMCh. 10 - Prob. PMCh. 10 - Prob. QMCh. 10 - Prob. RMCh. 10 - Prob. SMCh. 10 - Prob. TMCh. 10 - Prob. UMCh. 10 - Prob. VMCh. 10 - Prob. WMCh. 10 - Prob. XMCh. 10 - Prob. YMCh. 10 - Prob. ZMCh. 10 - Prob. 1MCCh. 10 - Prob. 2MCCh. 10 - Prob. 3MCCh. 10 - Prob. 4MCCh. 10 - Which radioactive decay mode does not result in a...Ch. 10 - Prob. 6MCCh. 10 - Prob. 7MCCh. 10 - Prob. 8MCCh. 10 - How many half-lives would it take for a sample of...Ch. 10 - Which of the following is not conserved in all...Ch. 10 - Prob. 11MCCh. 10 - Prob. 12MCCh. 10 - Prob. 13MCCh. 10 - Which unit is most closely associated with the...Ch. 10 - Prob. 15MCCh. 10 - Prob. 1FIBCh. 10 - Prob. 2FIBCh. 10 - The collective name for neutrons and protons in a...Ch. 10 - Prob. 4FIBCh. 10 - No stable nuclides exist that have Z greater than...Ch. 10 - Prob. 6FIBCh. 10 - The amount of a radioactive isotope will have...Ch. 10 - Prob. 8FIBCh. 10 - Prob. 9FIBCh. 10 - For an atomic bomb to explode, a(n) ____ mass is...Ch. 10 - In discussions of nuclear fusion reactions, the...Ch. 10 - Prob. 12FIBCh. 10 - Prob. 1SACh. 10 - Prob. 2SACh. 10 - Prob. 3SACh. 10 - Prob. 4SACh. 10 - Prob. 5SACh. 10 - What evidence is there to support the idea that...Ch. 10 - Prob. 7SACh. 10 - Prob. 8SACh. 10 - Prob. 9SACh. 10 - Prob. 10SACh. 10 - Prob. 11SACh. 10 - Prob. 12SACh. 10 - Prob. 13SACh. 10 - Prob. 14SACh. 10 - Prob. 15SACh. 10 - Prob. 16SACh. 10 - Prob. 17SACh. 10 - After three half-lives have gone by, what fraction...Ch. 10 - Prob. 19SACh. 10 - Prob. 20SACh. 10 - Prob. 21SACh. 10 - Prob. 22SACh. 10 - Prob. 23SACh. 10 - Prob. 24SACh. 10 - Prob. 25SACh. 10 - Prob. 26SACh. 10 - Prob. 27SACh. 10 - Prob. 28SACh. 10 - Prob. 29SACh. 10 - Prob. 30SACh. 10 - Prob. 31SACh. 10 - Prob. 32SACh. 10 - Prob. 33SACh. 10 - Prob. 34SACh. 10 - Prob. 35SACh. 10 - Prob. 36SACh. 10 - Prob. 37SACh. 10 - Prob. 38SACh. 10 - Prob. 39SACh. 10 - Prob. 40SACh. 10 - Prob. 41SACh. 10 - Prob. 42SACh. 10 - Visualize the connections and give the nuclear...Ch. 10 - Prob. 1AYKCh. 10 - The technique of carbon-14 dating relies on the...Ch. 10 - Prob. 3AYKCh. 10 - Prob. 4AYKCh. 10 - Prob. 5AYKCh. 10 - Fill in the nine gaps in this table.Ch. 10 - Fill in the nine gaps in this table.Ch. 10 - Prob. 3ECh. 10 - Prob. 4ECh. 10 - Prob. 5ECh. 10 - Prob. 6ECh. 10 - Prob. 7ECh. 10 - Actinium-225 (89225Ac) undergoes alpha decay. (a)...Ch. 10 - Prob. 9ECh. 10 - Prob. 10ECh. 10 - Prob. 11ECh. 10 - Prob. 12ECh. 10 - Prob. 13ECh. 10 - Prob. 14ECh. 10 - Prob. 15ECh. 10 - Prob. 16ECh. 10 - Prob. 17ECh. 10 - What is the half-life of thallium-206 if the...Ch. 10 - Use the graph in Fig. 10.24 to find the half-life...Ch. 10 - Prob. 20ECh. 10 - Prob. 21ECh. 10 - Prob. 22ECh. 10 - Prob. 23ECh. 10 - Prob. 24ECh. 10 - Prob. 25ECh. 10 - Prob. 26ECh. 10 - Prob. 27ECh. 10 - Prob. 28E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- What are the expected readings of the ammeter and voltmeter for the circuit in the figure below? (R = 5.60 Ω, ΔV = 6.30 V) ammeter I =arrow_forwardsimple diagram to illustrate the setup for each law- coulombs law and biot savart lawarrow_forwardA circular coil with 100 turns and a radius of 0.05 m is placed in a magnetic field that changes at auniform rate from 0.2 T to 0.8 T in 0.1 seconds. The plane of the coil is perpendicular to the field.• Calculate the induced electric field in the coil.• Calculate the current density in the coil given its conductivity σ.arrow_forward
- An L-C circuit has an inductance of 0.410 H and a capacitance of 0.250 nF . During the current oscillations, the maximum current in the inductor is 1.80 A . What is the maximum energy Emax stored in the capacitor at any time during the current oscillations? How many times per second does the capacitor contain the amount of energy found in part A? Please show all steps.arrow_forwardA long, straight wire carries a current of 10 A along what we’ll define to the be x-axis. A square loopin the x-y plane with side length 0.1 m is placed near the wire such that its closest side is parallel tothe wire and 0.05 m away.• Calculate the magnetic flux through the loop using Ampere’s law.arrow_forwardDescribe the motion of a charged particle entering a uniform magnetic field at an angle to the fieldlines. Include a diagram showing the velocity vector, magnetic field lines, and the path of the particle.arrow_forward
- Discuss the differences between the Biot-Savart law and Coulomb’s law in terms of their applicationsand the physical quantities they describe.arrow_forwardExplain why Ampere’s law can be used to find the magnetic field inside a solenoid but not outside.arrow_forward3. An Atwood machine consists of two masses, mA and m B, which are connected by an inelastic cord of negligible mass that passes over a pulley. If the pulley has radius RO and moment of inertia I about its axle, determine the acceleration of the masses mA and m B, and compare to the situation where the moment of inertia of the pulley is ignored. Ignore friction at the axle O. Use angular momentum and torque in this solutionarrow_forward
- A 0.850-m-long metal bar is pulled to the right at a steady 5.0 m/s perpendicular to a uniform, 0.650-T magnetic field. The bar rides on parallel metal rails connected through a 25-Ω, resistor (Figure 1), so the apparatus makes a complete circuit. Ignore the resistance of the bar and the rails. Please explain how to find the direction of the induced current.arrow_forwardFor each of the actions depicted, determine the direction (right, left, or zero) of the current induced to flow through the resistor in the circuit containing the secondary coil. The coils are wrapped around a plastic core. Immediately after the switch is closed, as shown in the figure, (Figure 1) in which direction does the current flow through the resistor? If the switch is then opened, as shown in the figure, in which direction does the current flow through the resistor? I have the answers to the question, but would like to understand the logic behind the answers. Please show steps.arrow_forwardWhen violet light of wavelength 415 nm falls on a single slit, it creates a central diffraction peak that is 8.60 cm wide on a screen that is 2.80 m away. Part A How wide is the slit? ΟΙ ΑΣΦ ? D= 2.7.10-8 Submit Previous Answers Request Answer × Incorrect; Try Again; 8 attempts remaining marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Half life | Radioactivity | Physics | FuseSchool; Author: FuseSchool - Global Education;https://www.youtube.com/watch?v=IDkNlU7zKYU;License: Standard YouTube License, CC-BY