
Concept explainers
(a)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
Oxidation of alcohol is the process in which the hydroxyl group of alcohol gets transformed either into carboxyl group or into an
(a)
Explanation of Solution
The given blank is: Can be oxidized to an aldehyde _______
Oxidation of alcohol is the process in which the hydroxyl group of alcohol gets transformed either into carboxyl group or into an aldehyde or to ketone. Product obtained may depend on the oxidizing agent used. Therefore, in presence of mild oxidizing agent, one gets aldehyde or ketone. In the presence of strong oxidizing agent,
Thus, Can be oxidized to an aldehyde:
(b)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
(b)
Explanation of Solution
The given blank is: Segment of a condensation polymer molecule _______.
The
Thus, segment of a condensation polymer molecule
(c)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
Oxidation of alcohol is the process in which the hydroxyl group of alcohol gets transformed either into carboxyl group or into an aldehyde or to ketone. Product obtained may depend on the oxidizing agent used. Therefore, in presence of mild oxidizing agent, one gets aldehyde or ketone.
(c)
Explanation of Solution
The given blank is: Can be oxidized to a carboxylic acid _____.
Oxidation of alcohol is the process in which the hydroxyl group of alcohol gets transformed either into carboxyl group or into an aldehyde or to ketone. Product obtained may depend on the oxidizing agent used. Therefore, in presence of mild oxidizing agent, one gets aldehyde or ketone. In the presence of strong oxidizing agent, carboxylic acid is obtained.
Thus, Can be oxidized to a carboxylic acid
(d)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
There are three types of alcohols: primary, secondary and tertiary. The type of alcohols depends upon the carbon atom to which the hydroxyl group is attached.
(d)
Explanation of Solution
The given blank is: A primary alcohol_____.
The compound given in Boxes A and G is a primary alcohol. The hydroxyl group is attached with a carbon atom that is attached with one carbon atom.
Thus, a primary alcohol
(e)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
The fatty acid can react with glycerol to form triglyceride with the formation of ester linkages. The reaction between acid and alcohol gives an ester as a major product. The ester group is derived by a carboxylic acid group in which the acidic hydrogen is replaced by an alkyl group.
(e)
Explanation of Solution
The given blank is: A triglyceride_____.
The triglyceride is formed by the reaction of glycerol and fatty acids. It is characterized by the presence of ester linkages. Box F contains a triglyceride.
Thus, A triglyceride
(f)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
Addition polymerization is the polymerization in which monomer units are linked together with no side product formation. Addition polymerization is given by the molecules which contains carbon-carbon double bond.
(f)
Explanation of Solution
The given blank is: Addition polymerization monomer_____.
Addition polymerization is given by the molecules which contains carbon-carbon double bond. Compounds in Box C contain a double bond.
Thus, addition polymerization monomer is
(g)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
An amide bond is formed between a carboxylic acid and an
(g)
Explanation of Solution
The given blank is: Contains an amide linkage_____.
An amide linkageis formed between a carboxylic acid and an amine. The carboxylic acid group of one amino acid reacts with an amino group of another amino acid to form an amide linkageby removing a water molecule. Compound in Box D and Box H contains an amide bond.
Thus, contains an amide linkage
(h)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
Oxidation of alcohol is the process in which the hydroxyl group of alcohol gets transformed either into an aldehyde or into ketone. To get an aldehyde or ketone, oxidation of acohol must takes place in the presence of mild oxidizing agent such as copper at 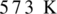 . Product obtained may be aldehyde or ketone. It depends on the nature of alcohol which may be primary, or secondary alcohol.
. Product obtained may be aldehyde or ketone. It depends on the nature of alcohol which may be primary, or secondary alcohol.
(h)
Explanation of Solution
The given blank is: Oxidized to form a ketone_____.
Oxidation of alcohol is the process in which the hydroxyl group of alcohol gets transformed either into an aldehyde or into ketone. Secondary alcohols undergo oxidation to form ketones. Compound in Box E and Box I are the secondary alcohols.
Thus, oxidized to form a ketone
(i)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
The reaction between acid and alcohol gives an ester as a major product. The ester is derived by a carboxylic acid group in which the acidic hydrogen is replaced by an alkyl group.
(i)
Explanation of Solution
The given blank is: Contains an ester linkage_____.
The triglyceride is formed by the reaction of glycerol and fatty acids. It is characterized by the presence of ester linkages. Box F contains a triglyceride.
Thus, contains an ester linkage
(j)
Interpretation:
The given blanks have to be filled with suitable words.
Concept Introduction:
Polymers are the high molecular mass compounds. The small monomers units joined together to form large molecule and this reaction is known as polymerization reaction. When two small molecules condense together to form a polymer with elimination of water molecule, the reaction is known as condensation polymerization.
(j)
Explanation of Solution
The given blank is: Condensation polymerization monomer_____.
Box B contains a compound in which two amine groupsare present. These two amine groups can undergo condensation polymerization which is characterized by the loss of hydrogen molecule.
Thus, Condensation polymerization monomer
Want to see more full solutions like this?
Chapter 10 Solutions
Bundle: Chemistry: The Molecular Science, 5th, Loose-Leaf + OWLv2 with Quick Prep 24-Months Printed Access Card
- Consider two elements, X and Z. Both have cubic-based unit cells with the same edge lengths. X has a bcc unit cell while Z has a fcc unit cell. Which of the following statements is TRUE? Group of answer choices Z has a larger density than X X has more particles in its unit cell than Z does X has a larger density than Z Z has a larger unit cell volume than Xarrow_forwardHow many particles does a face-centered cubic (fcc) unit cell contain? Group of answer choices 2 14 8 4arrow_forwardV Highlight all of the carbon atoms that have at least one beta (B) hydrogen, using red for one ẞ hydrogen, blue for two ẞ hydrogens, and green for three ẞ hydrogens. If none of the carbon atoms have ẞ hydrogens, check the box underneath the molecule. ED X None of the carbon atoms have ẞ hydrogens. Explanation esc 2 Check * F1 F2 1 2 80 # 3 Q W tab A caps lock shift fn control F3 N S option O 694 $ F4 F5 F6 005 % E R D F LL 6 olo 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility A DII F7 F8 87 & * 8 T Y U G H 4 F9 F10 ( 9 0 E F11 F12 உ J K L + || X C V B N M H H command option commandarrow_forward
- Consider the reaction below and answer the following questions. Part 1 of 4 Br NaOCH2CH3 Identify the mechanisms involved. Check all that apply. SN 1 SN 2 E1 E2 None of the above Part 2 of 4 Skip Part Check esc F1 F2 lock 1 2 Q W A S #3 80 F3 F4 F5 F6 Save For © 2025 McGraw Hill LLC. All Rights Reserved. Terms ˇˇ % & 4 5 6 89 7 IK A 分 བ F7 F8 F9 F * E R T Y U 8 9 D F G H K V B N M 0 Oarrow_forwardWhat kind of holes are not generated when solid-state particles adopt a close packing pattern? Group of answer choices tetrahedral cubic octahedral None of the other choices are correctarrow_forwardFor the reaction below: 1. Draw all reasonable elimination products to the right of the arrow. 2. In the box below the reaction, redraw any product you expect to be a major product. 田 Major Product: Check ☐ + I Na OH esc F1 F2 2 1 @ 2 Q W tab A caps lock S #3 80 F3 69 4 σ F4 % 95 S Click and drag to sta drawing a structure mm Save For Later 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use GO DII F5 F6 F7 F8 F9 F10 6 CO 89 & 7 LU E R T Y U 8* 9 0 D F G H J K L Z X C V B N M 36arrow_forward
- Problem 7 of 10 Draw the major product of this reaction. Ignore inorganic byproducts. S' S 1. BuLi 2. ethylene oxide (C2H4O) Select to Draw a Submitarrow_forwardFeedback (4/10) 30% Retry Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the reactant and missing intermediates involved in this reaction. Include all lone pairs and charges as appropriate. Ignore inorganic byproducts. Incorrect, 6 attempts remaining :0: Draw the Reactant H H3CO H- HIO: Ö-CH3 CH3OH2* protonation H. a H (+) H Ο CH3OH2 O: H3C protonation CH3OH deprotonation > CH3OH nucleophilic addition H. HO 0:0 Draw Intermediate a Xarrow_forwardCan I please get the blank spaces answered/answers?arrow_forward
- 1. Identify the following alkenes as E or Z NH₂ Br 2. Draw the structures based on the IUPAC names (3R,4R)-3-bromo-4-fluoro- 1-hexene (Z)-4-bromo-2-iodo-3-ethyl- 3-heptene تر 3. For the following, predict all possible elimination product(s) and circle the major product. HO H₂SO4 Heat 80 F4 OH H2SO4 Heat 어요 F5 F6 1 A DII 4 F7 F8 F9 % & 5 6 7 * ∞ 8 BAB 3 E R T Y U 9 F D G H J K O A F11 F10arrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. ○ O 1. H₂O, pyridine 2. neutralizing work-up a N W X 人 Parrow_forward✓ Check the box under each molecule that has a total of five ẞ hydrogens. If none of the molecules fit this description, check the box underneath the table. tab OH CI 0 Br xx Br None of these molecules have a total of five ẞ hydrogens. esc Explanation Check caps lock shift 1 fn control 02 F2 W Q A N #3 S 80 F3 E $ t 01 205 % 5 F5 & 7 © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility FT * 8 R T Y U כ F6 9 FIG F11 F D G H J K L C X V B < N M H option command P H + F12 commandarrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





