
Concept explainers
Predict the geometry of the following molecules and ion using the VSEPR model: (a) CBr4, (b) BCl3, (c) NF3, (d) H2Se, (e)
(a)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Answer to Problem 10.9QP
Tetrahedral geometry
Explanation of Solution
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (a)
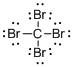
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 32.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 8 has to be subtracted with 32 as each bond contains two electrons with it and there are four bonds in the skeletal structure.
Finally, the 24 electrons got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (a) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral that is four atoms gets bonded with the central atom in the given molecule.
There exist no lone pair on carbon central atom then the molecular geometry for this molecule is tetrahedral.
(b)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Answer to Problem 10.9QP
(b)
Trigonal planar
Explanation of Solution
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (b)
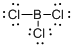
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 24.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 6 has to be subtracted with 24 as each bond contains two electrons with it and there are three bonds in the skeletal structure.
Finally, the 18 electrons got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (b) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type trigonal planar that is the boron atom contains three chlorine atoms and no lone pair of electrons over boron atoms hence the molecular geometry for the molecule is also trigonal planar.
(c)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Answer to Problem 10.9QP
Trigonal pyramidal
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (c)
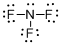
Explanation of Solution
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 26.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 6 has to be subtracted with 26 as each bond contains two electrons with it and there are three bonds in the skeletal structure.
Finally, the 20 electrons got after subtractions has to be equally distributed over all the atoms present in the molecule such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (c) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral since central atom nitrogen contains three fluorine atoms and one lone pair of electron.
The molecular geometry for the molecule is trigonal pyramidal because of one lone pair of electron it contain.
(d)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Answer to Problem 10.9QP
(d)
Bent shaped
Explanation of Solution
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (d)
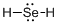
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 8.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 4 has to be subtracted with 8 as each bond contains two electrons with it and there are two bonds in the skeletal structure.
Finally, the 4 electrons got after subtractions has to be equally distributed over selenium atom such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (d) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral due to presence of two lone pair of electrons with it but the molecular geometry is bent since due to the presence of two lone pair of electrons over the central Se atom.
(e)
Interpretation: For the given set of molecules the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Answer to Problem 10.9QP
Bent shaped
Explanation of Solution
To predict: The geometry for the given molecule.
Draw the Lewis structure for the molecule (e)
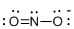
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 17 which is added with one electron due to the presence of charge -1 in the given molecule.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 6 has to be subtracted with 18 as each bond contains two electrons with it and there are three bonds in the skeletal structure.
Finally, the 12 electrons got after subtractions has to be equally distributed over the atoms such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (e) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type trigonal planar since there are two atoms and one lone pair electron around the central atom but the molecular geometry according to VSEPR theory is bent.
Want to see more full solutions like this?
Chapter 10 Solutions
Chemistry
- Indicate the products of the reaction between CH3COCH2COONa (Sodium acetoacetate) and BrCH2COOC2H5arrow_forwardIndicate whether the product of the reaction between Naphthalene and CrO3 in acetic acid at 25ºC is 1,4 naphthoquinone or phthalic anhydride.arrow_forwardIndicate the products of the reaction between CH3COCH2COOC2H5 and Na+-OC2H5.arrow_forward
- Primary, Secondary, and Tertiary Alcohols O-H O-H O-H R₁-C-H R₁-C-H R₁-C-R₁ H R₂ R₂ Primary Alcohol Secondary Alcohol ChemistryLearner.com R stands for Carbon group like ethyl methyl propyl Tertiary Alcohol If 1 carbon group with two H attached to alcoholic carbon, then primary If 2 carbon group and 1 H are attached to alcoholic carbon, then secondary IF 3 carbon group and no H attach to alcoholic carbon then tertiary. The bottom line Starting "Weak" oxidant material PCC, DMP, Swern, etc Primary alcohol Aldehyde OH Secondary alcohol Ketone OH "Strong" oxidant KMnO4, H₂CrO4 (or equivalent) OH Carboxylic acid 요 Ketone No reaction No reaction Tertiary alcohol 1. Is ethanol a primary, secondary, or tertiary alcohol? Write out the structures of ethanol and any oxidation products of ethanol. If there is more than one oxidation product, give the structure of each of the products. 2. Is 2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-propanol and any…arrow_forwardFormulate the reaction: Naphthalene with CrO3 in acetic acid at 25ºCarrow_forwardComplete the reaction hand written pleasearrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning



