
Concept explainers
Predict the geometries of the following ions: (a)
(a)
Interpretation:
For the given set of ions the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry:
It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
To predict: The geometry for the given molecule.
Explanation of Solution
Draw the Lewis structure for the molecule (a)
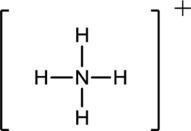
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 8.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 8 has to be subtracted with 8 as each bond contains two electrons with it and there are four bonds in the skeletal structure.
Finally, the structure does not have any electrons to be placed over the atoms since all the atoms have completed its valance shell.
Determine the molecular geometry for the molecule (a) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral since the central atom
There exist no lone pair on central atom hence the molecular geometry for this molecule is alsotetrahedral.
(b)
Interpretation:
For the given set of ions the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry:
It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
To predict: The geometry for the given molecule.
Explanation of Solution
Draw the Lewis structure for the molecule (b)
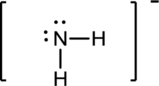
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 8.
The next step is to subtract the electrons present in the total number of bonds in the molecule with the total valence electrons such that 4 has to be subtracted with 8 as each bond contains two electrons with it and there are two bonds in the skeletal structure.
Finally, the 4 electrons got after subtractions has to be equally distributed such that each atom have completed valence shell.
Determine the molecular geometry for the molecule (b) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral.
Therefore, the molecular geometry for the given molecules is bent due to the presence of two lone pair of electrons with it.
(c)
Interpretation:
For the given set of ions the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry:
It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
To predict: The geometry for the given molecule.
Explanation of Solution
Draw the Lewis structure for the molecule (c)
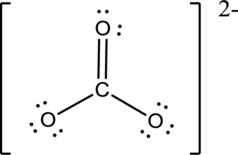
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 24.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 6 has to be subtracted with 24 as each bond contains two electrons with it and there are three bonds in the skeletal structure.
Finally, the 18 electrons got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell then considering the valency of carbon one
Determine the molecular geometry for the molecule (c) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type trigonal planar since there are 3 oxygen atoms bonded with carbon in
There exist no lone pair on central atom hence the molecular geometry for this molecule is also trigonal planar.
(d)
Interpretation:
For the given set of ions the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry:
It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Explanation of Solution
Draw the Lewis structure for the molecule (d)

First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 22 since it has one extra negative charge with it.
The next step is to subtract the electrons present in the total number of bonds in the molecule with the total valence electrons such that 4 has to be subtracted with 22 as each bond contains two electrons with it and there are two bonds in the skeletal structure.
Finally, the 18 electrons got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (d) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral due to the presence of two lone pairs.
Therefore, the molecular geometry for the given molecule is bent because of the repulsions produced by the two lone pairs present in the central atom.
(e)
Interpretation: For the given set of ions the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry: It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
To predict: The geometry for the given molecule.
Explanation of Solution
Draw the Lewis structure for the molecule (e)
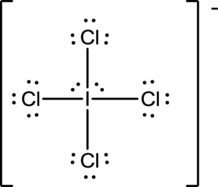
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 36.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 8 has to be subtracted with 36 as each bond contains two electrons with it and there are four bonds in the skeletal structure.
Finally, the 28 electrons got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (e) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type octahedral since there are four
Therefore, the geometry for the molecule is square planar due to the presence of two lone pair of electrons.
(f)
Interpretation:
For the given set of ions the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry:
It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
To predict: The geometry for the given molecule.
Explanation of Solution
Draw the Lewis structure for the molecule (f)
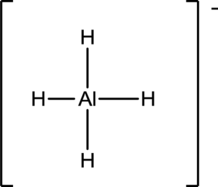
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 8 which include the presence one negative charge with it.
The next step is to subtract the electrons present in the total number of bonds in the molecule with the total valence electrons such that 8 has to be subtracted with 8 as each bond contains two electrons with it and there are four bonds in the skeletal structure.
Finally, there are no electrons to be placed over the atoms.
Determine the molecular geometry for the molecule (f) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral since there are four
There exist no lone pair on central atom hence the molecular geometry for this molecule is alsotetrahedral.
(g)
Interpretation:
For the given set of ions the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry:
It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
The geometry for the given molecule.
Explanation of Solution
Draw the Lewis structure for the molecule (g)
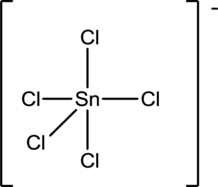
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 40 which include the presence one negative charge with it.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 10 has to be subtracted with 40 as each bond contains two electrons with it and there are five bonds in the skeletal structure.
Finally, the 30 electrons got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (g) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type trigonal bipyramidal since there are five chlorine atoms bonded with the central atom.
There exists no lone pair on central atom hence the molecular geometry for this molecule is also trigonalbipyramidal.
(h)
Interpretation:
For the given set of ions the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry:
It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
Explanation of Solution
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 8 which is included with the positive charge present in the given ion.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 6 has to be subtracted with 8 as each bond contains two electrons with it and there are three bonds in the skeletal structure.
Finally, the 2 electrons got after subtractions has to be equally distributed such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (h) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral since there are three
Therefore, the molecular geometry for the given ion is trigonal pyramidal since there exist one lone pair over O atom which is bonded with three hydrogen atoms.
(i)
Interpretation:
For the given set of ions the molecular geometry around the central metal should be predicted using VSEPR model.
Concept Introduction:
Molecular geometry:
It is defined as unique three dimensional arrangements of atoms around the central metal present in the molecule which is determined by using spectroscopic techniques and also by using Lewis structure or the valence shell electron pair repulsion theory (VSEPR).
VSEPR Theory:
As the name itself indicates that the basis for this theory is the electron pair that is bonded electron present in either single or double bonds or lone pair electrons, present in the valence shell tends to repel each other which then the tends to be in position in order to minimize the repulsions. The steps involved in the theory in describing the geometry is as follows,
- The first step is to draw the correct Lewis structure for the molecule.
- Then, the electron domain around the central atom should be counted and the geometry that matches with that type of domain in VSEPR should be determined.
- Finally, the geometry is predicted by using the orientation of atoms.
The molecules with considering the domains of type
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
Electron Domain: In VSEPR theory, both the lone pair and the bonded pair are together considered as electron domain regardless of the type of bond in which the bonded pair presents.
The geometry for the given molecule.
Explanation of Solution
Draw the Lewis structure for the molecule (i)
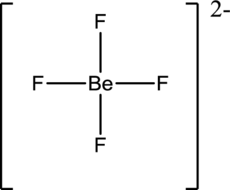
First the skeletal structure for the given molecule is drawn then the total number of valence electrons in the molecule is 32 which includes with the two negative charges present in the given ion.
The next step is to subtract the electrons present in the total number of bonds present in the molecule with the total valence electrons such that 8 has to be subtracted with 32 as each bond contains two electrons with it and there are four bonds in the skeletal structure.
Finally, the 24 electrons got after subtractions has to be equally distributed over the fluorine atoms present in the molecule such that each atom contains eight electrons in its valence shell.
Determine the molecular geometry for the molecule (i) using VSEPR.
The electron domain for the given molecule is obtained by viewing the Lewis structure which is of type tetrahedral since central atom does not contain any lone pair of electron with it.
The molecular geometry for the molecule is also tetrahedralas there isno lone pair of electrons present in the given ion.
Want to see more full solutions like this?
Chapter 10 Solutions
Chemistry
- Complete the mechanismarrow_forward8 00 6 = 10 10 Decide whether each of the molecules in the table below is stable, in the exact form in which it is drawn, at pH = 11. If you decide at least one molecule is not stable, then redraw one of the unstable molecules in its stable form below the table. (If more than unstable, you can pick any of them to redraw.) Check OH stable HO stable Ounstable unstable O OH stable unstable OH 80 F6 F5 stable Ounstable X Save For Later Sub 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy C ཀྭ་ A F7 매 F8 F9 4 F10arrow_forwardJust try completing it and it should be straightforward according to the professor and TAs.arrow_forward
- The grading is not on correctness, so if you can just get to the correct answers without perfectionism that would be great. They care about the steps and reasoning and that you did something. I asked for an extension, but was denied the extension.arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers. Something that looks reasonable or correct would be sufficient. If you can get many of them correct that would be great!arrow_forward
- Take a look at the following molecule, and then answer the questions in the table below it. (You can click the other tab to see the molecule without the colored regions.) with colored region plain 0= CH2-0-C-(CH2)16-CH3 =0 CH-O-C (CH2)7-CH=CH-(CH2)5-CH3 D CH3 | + OMPLO CH3-N-CH2-CH2-0-P-O-CH2 B CH3 A Try again * 000 Ar 8 0 ?arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forwardShow your work and do something that is reasonable. It does not have to be 100% correct. Just show something that looks good or pretty good as acceptable answers.arrow_forward
- = 1 = 2 3 4 5 6 ✓ 7 8 ✓ 9 =10 Devise a synthesis to prepare the product from the given starting material. Complete the following reaction scheme. Part 1 of 3 -Br Draw the structure for compound A. Check Step 1 Step 2 A Click and drag to start drawing a structure. × ↓m + OH Save For Later S 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privaarrow_forwardPredict the products of this organic reduction: 田 Check AP + + H2 Lindlar catalyst Click an drawing 2025 McGraw Hill LLC. All Rigarrow_forward70 Suppose the molecule below is in acidic aqueous solution. Is keto-enol tautomerization possible? • If a keto-enol tautomerization is possible, draw the mechanism for it. Be sure any extra reagents you add to the left-hand sid available in this solution. • If a keto-enol tautomerization is not possible, check the box under the drawing area. : ☐ Add/Remove step Click and drag to st drawing a structure Check Save For Late. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Usearrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning




