
Chemistry
12th Edition
ISBN: 9780078021510
Author: Raymond Chang Dr., Kenneth Goldsby Professor
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.66QP
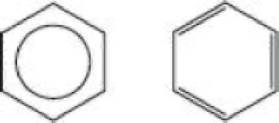
Explain why the symbol on the left is a better representation of benzene molecules than that on the right.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Hand written equations please
Hand written equations please
>
each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that:
1. the rate of substitution doesn't depend on nucleophile concentration and
2. the products are a roughly 50/50 mixture of enantiomers.
Substrate A
Substrate B
Faster Rate
X
Ś
CI
(Choose one)
(Choose one)
CI
Br
Explanation
Check
Br
(Choose one)
© 2025 McGraw Hill LLC. All Rights F
Chapter 10 Solutions
Chemistry
Ch. 10.1 - Use the VSEPR model to predict the geometry of (a)...Ch. 10.1 - Which of the following geometries has a greater...Ch. 10.2 - Does the AlCl3 molecule have a dipole moment?Ch. 10.2 - Carbon dioxide has a linear geometry and is...Ch. 10.3 - Compare the Lewis theory and the valence bond...Ch. 10.4 - Determine the hybridization state of the...Ch. 10.4 - Describe the hybridization state of Se in SeF6.Ch. 10.4 - What is the hybridization of Xe in XeF4Ch. 10.5 - Describe the bonding in the hydrogen cyanide...Ch. 10.5 - Which of the following pairs of atomic orbitals on...
Ch. 10.6 - One way to account for the fact that an O2...Ch. 10.7 - Estimate the bond enthalpy (kJ/mol) of the H2+...Ch. 10.7 - Which of the following species has a longer bond...Ch. 10.8 - Describe the bonding in the nitrate ion (NO3) in...Ch. 10 - How is the geometry of a molecule defined and why...Ch. 10 - Sketch the shape of a linear triatomic molecule, a...Ch. 10 - How many atoms are directly bonded to the central...Ch. 10 - Discuss the basic features of the VSEPR model....Ch. 10 - Prob. 10.5QPCh. 10 - Prob. 10.6QPCh. 10 - Predict the geometries of the following species...Ch. 10 - Predict the geometries of the following species:...Ch. 10 - Predict the geometry of the following molecules...Ch. 10 - Predict the geometry of the following molecules...Ch. 10 - Predict the geometry of the following molecules...Ch. 10 - Predict the geometries of the following ions: (a)...Ch. 10 - Describe the geometry around each of the three...Ch. 10 - Which of the following species are tetrahedral?...Ch. 10 - Prob. 10.15QPCh. 10 - Prob. 10.16QPCh. 10 - Prob. 10.17QPCh. 10 - The bonds in beryllium hydride (BeH2) molecules...Ch. 10 - Referring to Table 10.3, arrange the following...Ch. 10 - The dipole moments of the hydrogen halides...Ch. 10 - List the following molecules in order of...Ch. 10 - Does the molecule OCS have a higher or lower...Ch. 10 - Which of the molecules (a) or (b) has a higher...Ch. 10 - Prob. 10.24QPCh. 10 - What is valence bond theory? How does it differ...Ch. 10 - Use valence bond theory to explain the bonding in...Ch. 10 - Prob. 10.27QPCh. 10 - Prob. 10.28QPCh. 10 - What is the angle between the following two hybrid...Ch. 10 - How would you distinguish between a sigma bond and...Ch. 10 - Describe the bonding scheme of the AsH3 molecule...Ch. 10 - What is the hybridization state of Si in SiH4 and...Ch. 10 - Describe the change in hybridization (if any) of...Ch. 10 - Consider the reaction BF3+NH3F3BNH3 Describe the...Ch. 10 - What hybrid orbitals are used by nitrogen atoms in...Ch. 10 - What are the hybrid orbitals of the carbon atoms...Ch. 10 - Specify which hybrid orbitals are used by carbon...Ch. 10 - Prob. 10.38QPCh. 10 - The allene molecule H2CCCH2 is linear (the three C...Ch. 10 - Prob. 10.40QPCh. 10 - How many sigma bonds and pi bonds are there in...Ch. 10 - How many pi bonds and sigma bonds are there in the...Ch. 10 - Give the formula of a cation comprised of iodine...Ch. 10 - Give the formula of an anion comprised of iodine...Ch. 10 - What is molecular orbital theory? How does it...Ch. 10 - Sketch the shapes of the following molecular...Ch. 10 - 10.47 Compare the Lewis theory, valence bond...Ch. 10 - Explain the significance of bond order. Can bond...Ch. 10 - Explain in molecular orbital terms the changes in...Ch. 10 - The formation of H2 from two H atoms is an...Ch. 10 - Prob. 10.51QPCh. 10 - Arrange the following species in order of...Ch. 10 - Prob. 10.53QPCh. 10 - Which of these species has a longer bond, B2 or...Ch. 10 - Acetylene (C2H2) has a tendency to lose two...Ch. 10 - Compare the Lewis and molecular orbital treatments...Ch. 10 - Explain why the bond order of N2 is greater than...Ch. 10 - Compare the relative stability of the following...Ch. 10 - Use molecular orbital theory to compare the...Ch. 10 - A single bond is almost always a sigma bond, and a...Ch. 10 - In 2009 the ion N23 was isolated. Use a molecular...Ch. 10 - The following potential energy curve represents...Ch. 10 - Prob. 10.63QPCh. 10 - Prob. 10.64QPCh. 10 - Prob. 10.65QPCh. 10 - Explain why the symbol on the left is a better...Ch. 10 - Determine which of these molecules has a more...Ch. 10 - Nitryl fluoride (FNO2) is very reactive...Ch. 10 - Describe the bonding in the nitrate ion NO3 in...Ch. 10 - Prob. 10.70QPCh. 10 - Which of the following species is not likely to...Ch. 10 - Draw the Lewis structure of mercury(II) bromide....Ch. 10 - Sketch the bond moments and resultant dipole...Ch. 10 - Although both carbon and silicon are in Group 4A,...Ch. 10 - Acetaminophen is the active ingredient in Tylenol....Ch. 10 - Caffeine is a stimulant drug present in coffee....Ch. 10 - Predict the geometry of sulfur dichloride (SCl2)...Ch. 10 - Antimony pentafluoride, SbF5, reacts with XeF4 and...Ch. 10 - Draw Lewis structures and give the other...Ch. 10 - Predict the bond angles for the following...Ch. 10 - Briefly compare the VSEPR and hybridization...Ch. 10 - Describe the hybridization state of arsenic in...Ch. 10 - Draw Lewis structures and give the other...Ch. 10 - Which of the following molecules and ions are...Ch. 10 - Prob. 10.85QPCh. 10 - The N2F2 molecule can exist in either of the...Ch. 10 - Cyclopropane (C3H6) has the shape of a triangle in...Ch. 10 - The compound 1,2-dichloroethane (C2H4Cl2) is...Ch. 10 - Does the following molecule have a dipole moment?...Ch. 10 - So-called greenhouse gases, which contribute to...Ch. 10 - The bond angle of SO2 is very close to 120, even...Ch. 10 - 3-azido-3-deoxythymidine, shown here, commonly...Ch. 10 - The following molecules (AX4Y2) all have...Ch. 10 - The compounds carbon tetrachloride (CCl4) and...Ch. 10 - Prob. 10.95QPCh. 10 - What are the hybridization states of the C and N...Ch. 10 - Use molecular orbital theory to explain the...Ch. 10 - Referring to the Chemistry in Action essay...Ch. 10 - Which of the molecules (a)(c) are polar?Ch. 10 - Prob. 10.100QPCh. 10 - The stable allotropic form of phosphorus is P4, in...Ch. 10 - Referring to Table 9.4, explain why the bond...Ch. 10 - Use molecular orbital theory to explain the...Ch. 10 - The ionic character of the bond in a diatomic...Ch. 10 - Prob. 10.105QPCh. 10 - Prob. 10.106QPCh. 10 - Aluminum trichloride (AlCl3) is an...Ch. 10 - The molecules cis-dichloroethylene and...Ch. 10 - Prob. 10.109QPCh. 10 - Prob. 10.110QPCh. 10 - The molecule benzyne (C6H4) is a very reactive...Ch. 10 - Assume that the third-period element phosphorus...Ch. 10 - Consider a N2 molecule in its first excited...Ch. 10 - Prob. 10.114QPCh. 10 - Prob. 10.116QPCh. 10 - Draw the Lewis structure of ketene (C2H2O) and...Ch. 10 - TCDD, or 2,3,7,8-tetrachlorodibenzo-p-dioxin, is a...Ch. 10 - Write the electron configuration of the cyanide...Ch. 10 - Prob. 10.120QPCh. 10 - The geometries discussed in this chapter all lend...Ch. 10 - Prob. 10.122QPCh. 10 - Which of the following ions possess a dipole...Ch. 10 - Given that the order of molecular orbitals for NO...Ch. 10 - Shown here are molecular models of SX4 for X = F,...Ch. 10 - Based on what you have learned from this chapter...Ch. 10 - How many carbon atoms are contained in one square...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- NMR spectrum of ethyl acetate has signals whose chemical shifts are indicated below. Which hydrogen or set of hydrogens corresponds to the signal at 4.1 ppm? Select the single best answer. The H O HỌC—C—0—CH, CH, 2 A ethyl acetate H NMR: 1.3 ppm, 2.0 ppm, 4.1 ppm Check OA B OC ch B C Save For Later Submit Ass © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center |arrow_forwardHow many signals do you expect in the H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red Note for advanced students: In this question, any multiplet is counted as one signal. 1 Number of signals in the 'H NMR spectrum. For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. Check For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. O ✓ No additional Hs to color in top molecule ง No additional Hs to color in bottom…arrow_forwardin the kinetics experiment, what were the values calculated? Select all that apply.a) equilibrium constantb) pHc) order of reactiond) rate contstantarrow_forward
- true or false, given that a 20.00 mL sample of NaOH took 24.15 mL of 0.141 M HCI to reach the endpoint in a titration, the concentration of the NaOH is 1.17 M.arrow_forwardin the bromothymol blue experiment, pKa was measured. A closely related compound has a Ka of 2.10 x 10-5. What is the pKa?a) 7.1b) 4.7c) 2.0arrow_forwardcalculate the equilibrium concentration of H2 given that K= 0.017 at a constant temperature for this reaction. The inital concentration of HBr is 0.050 M.2HBr(g) ↔ H2(g) + Br2(g)a) 4.48 x 10-2 M b) 5.17 x 10-3 Mc) 1.03 x 10-2 Md) 1.70 x 10-2 Marrow_forward
- true or falsegiven these two equilibria with their equilibrium constants:H2(g) + CI2(l) ↔ 2HCI(g) K= 0.006 CI2(l) ↔ CI2(g) K= 0.30The equilibrium contstant for the following reaction is 1.8H2(g) + CI2 ↔ 2HCI(g)arrow_forwardI2(g) + CI2(g) ↔ 2ICIK for this reaction is 81.9. Find the equilibrium concentration of I2 if the inital concentration of I2 and CI2 are 0.010 Marrow_forwardtrue or false,the equilibrium constant for this reaction is 0.50.PCI5(g) ↔ PCI3(g) + CI2(g)Based on the above, the equilibrium constant for the following reaction is 0.25.2PCI5(g) ↔. 2PCI3(g) + 2CI2(g)arrow_forward
- true or false, using the following equilibrium, if carbon dioxide is added the equilibrium will shift toward the productsC(s) + CO2(g) ↔ 2CO(g)arrow_forward2S2O2/3- (aq) + I2 (aq) ---> S4O2/6- (aq) +2I- (aq) Experiment I2 (M) S2O3- (M) Initital Rate (M/s) 1 0.01 0.01 0.0004 2 0.01 0.02 0.0004 3 0.02 0.01 0.0008 Calculate the overall order for this reaction using the table data a) 3b) 0c) 2d) 1arrow_forwardthe decomposition of N2O5 is the first order with a half-life of 1.98 minutes. If the inital concentration of N2O5 is 0.200 M, what is the concentration after 6 minutes?a) 0.612 Mb) 0.035 Mc) 0.024 Md) 0.100 Marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY