
Concept explainers
(a)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
The mechanism for epoxides under neutral or basic conditions consists of an
Answer to Problem 10.53P
The product of the given reaction is:
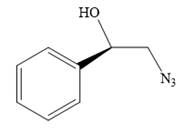
Explanation of Solution
The given reaction is:
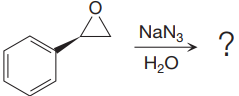
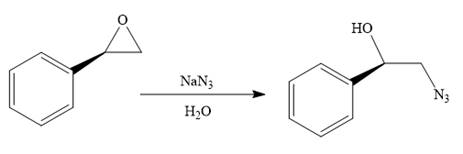
When the given epoxide is treated with sodium azide (basic conditions), the azide ion in sodium azide acts as a nucleophile and attacks the less substituted carbon atom (highlighted in red) of the epoxide. Due to this, the highly strained epoxide ring opens and the azide gets attached to the less substituted carbon atom. In the presence of a solvent such as water, the negatively charged oxygen atom is protonated resulting in the final product. The product of the reaction when the given epoxide is treated with sodium azide is as below:
Epoxides can undergo nucleophilic substitution reactions under neutral or basic conditions in which the nucleophile attacks the least substituted carbon atom in the epoxide.
(b)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
The mechanism for the epoxides under neutral or basic conditions consists of an
Answer to Problem 10.53P
The product of the given reaction is:
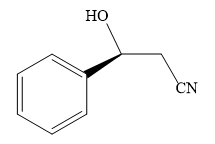
Explanation of Solution
The given reaction is:
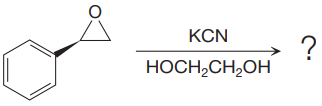
The given reaction conditions are basic as the potassium cyanide is a weak base and nucleophile.
When the given epoxide is treated with potassium cyanide (neutral conditions), the cyanide ion acts as a nucleophile and attacks the least substituted carbon atom of the epoxide. Due to this, the highly strained epoxide ring opens and the cyanide ion gets attached to the carbon. In the presence of a solvent such as ethanediol, the negatively charged oxygen atom is protonated resulting in the final product. The product of the reaction when the given epoxide is treated with sodium azide is as below:
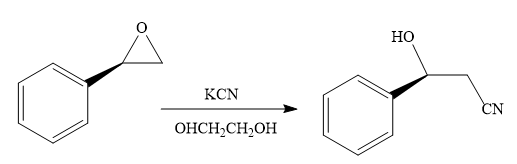
Epoxides can undergo nucleophilic substitution reactions under neutral or basic conditions in which the nucleophile attacks the least substituted carbon atom in the epoxide.
(c)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
Under acidic conditions, the first step is the protonation of
Answer to Problem 10.53P
The product of the given reaction is:
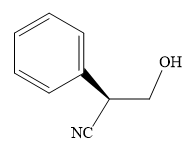
Explanation of Solution
The given reaction is:
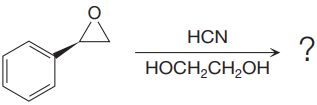
The given reaction conditions are acidic as hydrogen cyanide is a weak acid.
When the given epoxide is treated with hydrogen cyanide (acidic conditions), the first step is the protonation of the epoxide oxygen atom. Due to this, a partial positive charge is generated on both carbon atoms in the epoxide. Out of the two carbon atoms, the one that is most substituted would be able to stabilize the partially developed charge, and thus, in the second step, the nucleophile, cyanide ion attacks the most substituted carbon atom from the side opposite to the
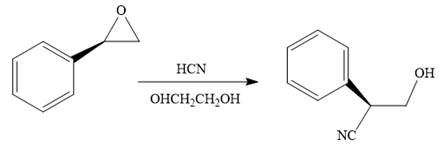
Epoxides can undergo nucleophilic substitution reactions under acidic conditions in which the nucleophile attacks the most substituted carbon atom in the epoxide.
(d)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
The mechanism for the epoxides under neutral or basic conditions consists of an
Answer to Problem 10.53P
The product of the given reaction is:
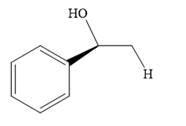
Explanation of Solution
The given reaction is:
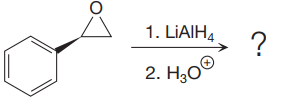
The given reaction conditions are basic as lithium aluminum hydride is a strong base.
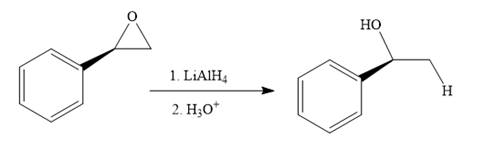
Epoxides can undergo nucleophilic substitution reactions under neutral or basic conditions in which the nucleophile attacks the least substituted carbon atom in the epoxide.
(e)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
Under acidic conditions, the first step is the protonation the
Answer to Problem 10.53P
The product of the given reaction is:
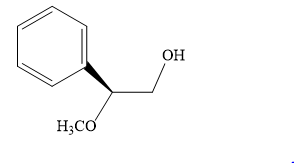
Explanation of Solution
The given reaction is:
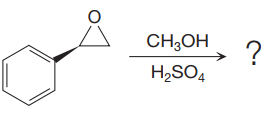
The given reaction conditions are methanol in sulfuric acid. Sulfuric acid is a strong acid. When it is treated with methanol, it will produce protonated methanol which is a strongest known acid. When the given epoxide is treated with protonated methanol, (highly acidic conditions), the first step is the protonation of the oxygen atom in the epoxide. Due to this, a partial positive charge is generated on both the carbon atoms in the epoxide. Out of the two carbon atoms, the one that is most substituted would be able to stabilize the partially developed positive charge. Thus, in the second step, the nucleophile, methoxide ion attacks the most substituted carbon atom from the side opposite to the protonated
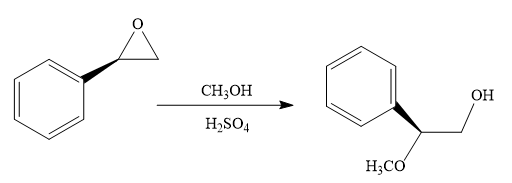
Epoxides can undergo nucleophilic substitution reactions under acidic conditions in which the nucleophile attacks on the most substituted carbon atom in the epoxide.
(f)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
The mechanism for the epoxides under neutral or basic conditions consists of an
Answer to Problem 10.53P
The product of the given reaction is:
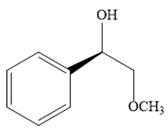
Explanation of Solution
The given reaction is:
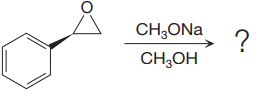
When the given epoxide is treated with sodium methoxide in methanol (basic conditions), the methoxide ion acts as a nucleophile and attacks the least substituted carbon atom of the epoxide. Due to this, the highly strained epoxide ring opens and the methoxide ion gets attached to the least substituted carbon atom. The product of the reaction when the given epoxide is treated with sodium methoxide in methanol is as below:
Epoxides can undergo nucleophilic substitution reactions under neutral or basic conditions in which the nucleophile attacks the least substituted carbon atom in the epoxide.
(g)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
The mechanism for the epoxides under neutral or basic conditions consists of an
Answer to Problem 10.53P
The product of the given reaction is:
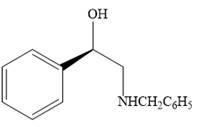
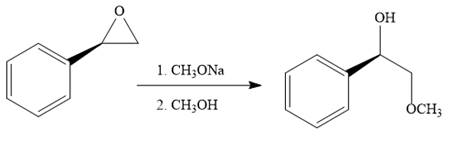
Explanation of Solution
The given reaction is:
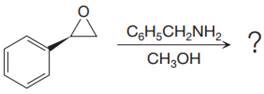
When the given epoxide is treated with benzyl
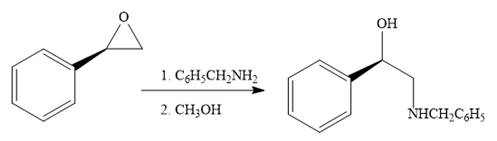
Epoxides can undergo nucleophilic substitution reactions under neutral or basic conditions in which the nucleophile attacks on the least substituted carbon atom in the epoxide.
(h)
Interpretation:
The product of the given reaction is to be predicted.
Concept introduction:
Epoxides react readily under neutral, basic, or acidic conditions. Epoxides can undergo
The mechanism for the epoxides under neutral or basic conditions consists of an
Answer to Problem 10.53P
The product of the given reaction is:
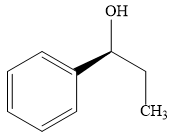
Explanation of Solution
The given reaction is:
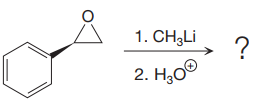
Methyl lithium is an inorganic methylating agent. When the given epoxide is treated with methyl lithium (basic conditions), the methyl anion acts as a nucleophile and attacks the least substituted carbon atom of the epoxide. Due to this, the highly strained epoxide ring opens. In the second step, acidic workup is important so as to protonate the negatively charged oxygen atom.
The product of the reaction when the given epoxide is treated with methyl lithium in the acidic workup is as below:
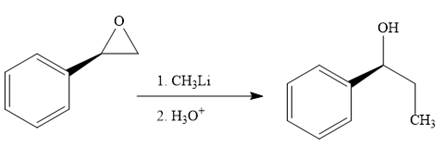
Epoxides can undergo nucleophilic substitution reactions under neutral or basic conditions in which the nucleophile attacks the least substituted carbon atom in the epoxide.
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- CH, CH CH₂ CH₂ Phytyl side chain 5. What is the expected order of elution of compounds A-D below from a chromatography column packed with silica gel, eluting with hexane/ethyl acetate? C D OHarrow_forwardPlease analze my gel electrophoresis column of the VRK1 kinase (MW: 39.71 kDa). Attached is the following image for the order of column wells and my gel.arrow_forward2.0arrow_forward
- Write the electron configuration of an atom of the element highlighted in this outline of the Periodic Table: 1 23 4 5 6 7 He Ne Ar Kr Xe Rn Hint: you do not need to know the name or symbol of the highlighted element! ☐arrow_forwardCompare these chromatograms of three anti-psychotic drugs done by HPLC and SFC. Why is there the difference in separation time for SFC versus HPLC? Hint, use the Van Deemter plot as a guide in answering this question. Why, fundamentally, would you expect a faster separation for SFC than HPLC, in general?arrow_forwardA certain inorganic cation has an electrophoretic mobility of 5.27 x 10-4 cm2s-1V-1. The same ion has a diffusion coefficient of 9.5 x 10-6cm2s-1. If this ion is separated from cations by CZE with a 75cm capillary, what is the expected plate count, N, at an applied voltage of 15.0kV? Under these separation conditions, the electroosmotic flow rate was 0.85mm s-1 toward the cathode. If the detector was 50.0cm from the injection end of the capillary, how long would it take in minutes for the analyte cation to reach the detector after the field was applied?arrow_forward
- 2.arrow_forwardPlease solve for the following Electrochemistry that occursarrow_forwardCommercial bleach contains either chlorine or oxygen as an active ingredient. A commercial oxygenated bleach is much safer to handle and less likely to ruin your clothes. It is possible to determine the amount of active ingredient in an oxygenated bleach product by performing a redox titration. The balance reaction for such a titration is: 6H+ +5H2O2 +2MnO4- à 5O2 + 2Mn2+ + 8H2O If you performed the following procedure: “First, dilute the Seventh Generation Non-Chlorine Bleach by pipetting 10 mL of bleach in a 100 mL volumetric flask and filling the flask to the mark with distilled water. Next, pipet 10 mL of the diluted bleach solution into a 250 mL Erlenmeyer flask and add 20 mL of 1.0 M H2SO4 to the flask. This solution should be titrated with 0.0100 M KMnO4 solution.” It took 18.47mL of the KMnO4 to reach the endpoint on average. What was the concentration of H2O2 in the original bleach solution in weight % assuming the density of bleach is 1g/mL?arrow_forward
- 10.arrow_forwardProper care of pH electrodes: Why can you not store a pH electrode in distilled water? What must you instead store it in? Why?arrow_forwardWrite the electron configuration of an atom of the element highlighted in this outline of the Periodic Table: 1 23 4 569 7 He Ne Ar Kr Xe Rn Hint: you do not need to know the name or symbol of the highlighted element! §arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

