![OWLv2 for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 1 term (6 months)](https://s3.amazonaws.com/compass-isbn-assets/textbook_empty_images/large_textbook_empty.svg)
OWLv2 for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 1 term (6 months)
11th Edition
ISBN: 9781305673939
Author: Darrell Ebbing; Steven D. Gammon
Publisher: Cengage Learning US
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 10, Problem 10.47QP
What hybrid orbitals would be expected for the central atom in each of the following molecules or ions?
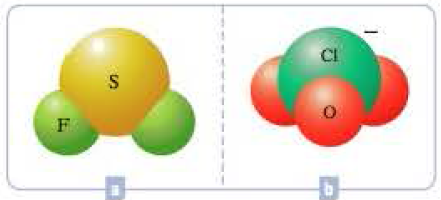
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
If a radiation intensity l0 = 2.5x1010 fotones s-1 cm2 causes a dissolución and an absorbance of 0.95 will be recorded. How much incident radiation is absorbed by the music screen?
From the causes of the detection of a spectral band of a spectrum obtained by a signal in the gaseous phase that is indicated, you can avoid or minimize those that have their origin in:a) the Doppler effectb) collisionsc) the life time of the excited state
a) Why is it possible that all types of atoms occupy the fundamental energy level?b) What should be the value of the participation function so that it occurs?c) keep in mind that the translational levels of a system are very close, which must be the condition that tenga lugar el condensado de átomos en el fundamental level?
Chapter 10 Solutions
OWLv2 for Ebbing/Gammon's General Chemistry, 11th Edition, [Instant Access], 1 term (6 months)
Ch. 10.1 - An atom in a molecule is surrounded by four pairs...Ch. 10.1 - Use the VSEPR method to predict the geometry of...Ch. 10.1 - Prob. 10.2ECh. 10.2 - Bromine trifluoride, BrF3, has a nonzero dipole...Ch. 10.2 - Which of the following would be expected to have a...Ch. 10.2 - Two molecules, each with the general formula AX3,...Ch. 10.3 - Using hybrid orbitals, describe the bonding in NH3...Ch. 10.4 - Describe the bonding on the carbon atom in carbon...Ch. 10.4 - Dinitrogen difluoride (see Example 10.5) exists as...Ch. 10.4 - Prob. 10.3CC
Ch. 10.6 - The C2 molecule exists in the vapor phase over...Ch. 10.6 - Give the orbital diagram and electron...Ch. 10 - Describe the main features of the VSEPR model.Ch. 10 - According to the VSEPR model, what are the...Ch. 10 - Why is a lone pair expected to occupy an...Ch. 10 - Prob. 10.4QPCh. 10 - Explain why nitrogen trifluoride has a small...Ch. 10 - Prob. 10.6QPCh. 10 - What is the angle between two sp3 hybrid orbitals?Ch. 10 - Prob. 10.8QPCh. 10 - Prob. 10.9QPCh. 10 - How does the valence bond description of a...Ch. 10 - Prob. 10.11QPCh. 10 - What factors determine the strength of interaction...Ch. 10 - Prob. 10.13QPCh. 10 - Prob. 10.14QPCh. 10 - Prob. 10.15QPCh. 10 - Describe the bonding in O3, using molecular...Ch. 10 - Prob. 10.17QPCh. 10 - Which of the following molecular geometries does...Ch. 10 - Which of the following would be a polar molecule?...Ch. 10 - Prob. 10.20QPCh. 10 - Best Lewis Formula and Molecular Geometry A...Ch. 10 - Prob. 10.22QPCh. 10 - Prob. 10.23QPCh. 10 - Which of the following molecular models correctly...Ch. 10 - Prob. 10.25QPCh. 10 - Prob. 10.26QPCh. 10 - Indicate what hybrid orbital depicted below is...Ch. 10 - An atom in a molecule has two bonds to other atoms...Ch. 10 - Two compounds have the same molecular formula,...Ch. 10 - A neutral molecule is identified as a...Ch. 10 - Acetic acid, the sour constituent of vinegar, has...Ch. 10 - What are the bond angles predicted by the VSEPR...Ch. 10 - Predict the shape or geometry of the following...Ch. 10 - Use the electron-pair repulsion model to predict...Ch. 10 - Predict the geometry of the following ions, using...Ch. 10 - Use the VSEPR model to predict the geometry of the...Ch. 10 - For each of the following molecules, state the...Ch. 10 - For each of the following molecules, state the...Ch. 10 - Prob. 10.39QPCh. 10 - From the electron-pair repulsion model, predict...Ch. 10 - Predict the geometries of the following ions,...Ch. 10 - Name the geometries expected for the following...Ch. 10 - a The molecule AsF3 has a dipole moment of 2.59 D....Ch. 10 - a The molecule BrF3 has a dipole moment of 1.19 D....Ch. 10 - Which of the following molecules would be expected...Ch. 10 - Which of the following molecules would be expected...Ch. 10 - What hybrid orbitals would be expected for the...Ch. 10 - What hybrid orbitals would be expected for the...Ch. 10 - What hybrid orbitals would be expected for the...Ch. 10 - What hybrid orbitals would be expected for the...Ch. 10 - a Mercury(II) chloride dissolves in water to give...Ch. 10 - a Nitrogen trifluoride, NF3, is a relatively...Ch. 10 - a Carbonyl fluoride, COF2, is an extremely...Ch. 10 - a The molecule HNNH exists as a transient species...Ch. 10 - The hyponitrite ion, ONNO, exists in solid...Ch. 10 - Fumaric acid, C4H4O4, occurs in the metabolism of...Ch. 10 - Describe the electronic structure of each of the...Ch. 10 - Use molecular orbital theory to describe the...Ch. 10 - Prob. 10.59QPCh. 10 - Write the molecular orbital configuration of the...Ch. 10 - Predict the molecular geometry of the following: a...Ch. 10 - Prob. 10.62QPCh. 10 - Which of the following molecules or ions are...Ch. 10 - Which of the following molecules or ions are...Ch. 10 - Describe the hybrid orbitals used by each carbon...Ch. 10 - Prob. 10.66QPCh. 10 - Explain how the dipole moment could be used to...Ch. 10 - Two compounds have the formula Pt(NH3)2Cl2....Ch. 10 - Explain in terms of bonding theory why all four...Ch. 10 - Explain in terms of bonding theory why all atoms...Ch. 10 - What is the molecular orbital configuration of...Ch. 10 - Prob. 10.72QPCh. 10 - Calcium carbide, CaC2, consists of Ca2+ and C22...Ch. 10 - Sodium peroxide, Na2O2, consists of Na+ and O22...Ch. 10 - The oxygen oxygen bond in O2 is 112 pm and in O2...Ch. 10 - The nitrogennitrogen bond distance in N2 is 109...Ch. 10 - Using molecular orbital theory, determine the...Ch. 10 - The ionization energy of O2 is smaller than the...Ch. 10 - Prob. 10.79QPCh. 10 - Prob. 10.80QPCh. 10 - Prob. 10.81QPCh. 10 - Prob. 10.82QPCh. 10 - What is the biological importance of stratospheric...Ch. 10 - Prob. 10.84QPCh. 10 - Prob. 10.85QPCh. 10 - The bond length in C2 is 131 pm. Compare this with...Ch. 10 - Calcium carbide, CaC2, has an ionic structure with...Ch. 10 - Write Lewis formulas for the BF molecule (two with...Ch. 10 - Boron trifluoride, BF3, reacts with ammonia, NH3,...Ch. 10 - Prob. 10.90QPCh. 10 - Allene (1,2-propadicne), a gas, has the following...Ch. 10 - Prob. 10.92QPCh. 10 - The triiodide ion, I3, and the azide ion, N3, have...Ch. 10 - Hydrogen azide (also known as hydrazoic acid),...Ch. 10 - Prob. 10.95QPCh. 10 - A molecule XF6 (having no lone pairs) has a dipole...Ch. 10 - Describe the molecular orbital configurations of...Ch. 10 - Prob. 10.98QPCh. 10 - Three different compounds have the same molecular...Ch. 10 - Prob. 10.100QPCh. 10 - Prob. 10.101QPCh. 10 - Solid sulfur normally consists of crystals of S8...Ch. 10 - Prob. 10.103QPCh. 10 - Consider the bonding in nitrate ion, NO3. First...Ch. 10 - A molecular compound is composed of 52.5% Xe,...Ch. 10 - A molecular compound is composed of 58.8% Xe,...Ch. 10 - A compound of chlorine and fluorine. ClFn, reacts...Ch. 10 - Excess fluorine, F2(g), reacts at 150C with...Ch. 10 - Prob. 10.109QPCh. 10 - One resonance formula of benzene, C6H6, is What is...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At the polar moment of Rnm transition, you can confirm thata) nunca can be ser 0b) is a very important magnitude in Raman spectroscopyc) is related to the probability of spectroscopic transactionsd) is related to the selection rulesarrow_forwardIn Fourier transformed spectroscopya) use a very sensitive monocromador systemb) the detection time is inferior to conventional spectroscopiac) the signal is detected depending on the frequencyd) occurs simultaneously at all frequency intervalsarrow_forwardIf a radiation intensity l0 = 2.5x1010 fotones s-1cm2 results in a dissolución, an absorption of 0.95 will be recorded. What is the percentage of incident radiation and transmission?a) 88.88% b) 5% c) 11.22% d) 95%arrow_forward
- Indicate the spectroscopic transmission that requires greater energy radiation. Justification:a) NMR b) vibration c) electronica d) rotationarrow_forwardAfter an induced absorption process of an intensity, there are (without population inversion) transitions between:a) vibrational and rotational levels in the infrared region, we obtainb) vibrational levels exclusively in the infrared regionc) vibrational and rotational levels in the microwave regiond) transitions between nuclear spin levels in the radio frequency regionarrow_forwardIn a spontaneous emission process:a) the ground state population decreasesb) the excited state population decreasesc) the non-radiative component is predominantd) the emitted radiation is coherentarrow_forward
- For a molecule there are 3 energy levels A, B and C, where B is an intermediate energy level between A and C. The A → C transition occurs at 480 nm and the B → C transition occurs at 885 nm. Indicate the wavelength at which the A → B transition will occur.arrow_forwardFor a molecule there are three energy levels: A, B and C. If the transition A → B occurs at 1049 nm and the transition B → C occurs at 885 nm, we can say that the wavelength of the transition A → C will occur at approximately:a) 164 nm b) 1934 nm c) 480 nm d) 967 nmarrow_forward: Naming the Alkanes a) Write the IUPAC nomenclature of the compound below b) Draw 4-isopropyl-2,4,5-trimethylheptane, identify the primary, secondary, tertiary, and quaternary carbons. c) Rank pentane, neopentane and isopentane for boiling point. pentane: H3C-CH2-CH2-CH2-CH3 neopentane: CH3 H3C-C-CH3 isopentane: CH3 CH3 H3C-CH2-CH-CH3arrow_forward
- An essential part of the experimental design process is to select appropriate dependent and independent variables. True Falsearrow_forward10.00 g of Compound X with molecular formula C₂Hg are burned in a constant-pressure calorimeter containing 40.00 kg of water at 25 °C. The temperature of the water is observed to rise by 2.604 °C. (You may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) Calculate the standard heat of formation of Compound X at 25 °C. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.arrow_forwardneed help not sure what am doing wrong step by step please answer is 971A During the lecture, we calculated the Debye length at physiological salt concentrations and temperature, i.e. at an ionic strength of 150 mM (i.e. 0.150 mol/l) and a temperature of T=310 K. We predicted that electrostatic interactions are effectively screened beyond distances of 8.1 Å in solutions with a physiological salt concentration. What is the Debye length in a sample of distilled water with an ionic strength of 10.0 µM (i.e. 1.00 * 10-5 mol/l)? Assume room temperature, i.e. T= 298 K, and provide your answer as a numerical expression with 3 significant figures in Å (1 Å = 10-10 m).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY