
Concept explainers
Give the IUPAC name for each compound.
a. 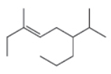 c.
c. 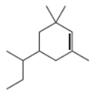 e.
e. 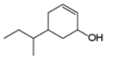
b. 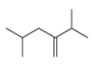 d.
d. 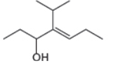 f.
f. 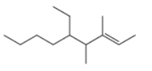
(a)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 10.38P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
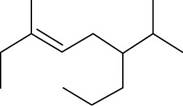
Figure 1
The numbering of carbon atoms present in the longest carbon chain is shown below.
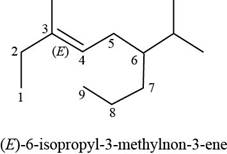
Figure 2
The longest carbon chain has nine carbon atoms. The root word used for nine carbon atoms is non and the suffix used for
The IUPAC name for the given compound is
(b)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 10.38P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
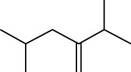
Figure 3
The numbering of carbon atoms present in longest chain of the given compound is shown below.
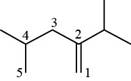
Figure 4
The longest carbon-carbon chain has five carbon atoms. The root word used for five carbon atoms is pent and the suffix used for
The IUPAC name for the given compound is
(c)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 10.38P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
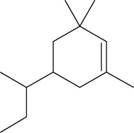
Figure 5
The numbering of carbon atoms present in longest carbon chain of the given compound is shown below.
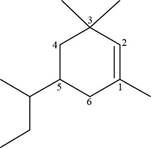
Figure 6
The longest carbon chain has six carbon atoms. The root word used for six carbon atoms is hex and the suffix used for
The IUPAC name for the given compound is
(d)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 10.38P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
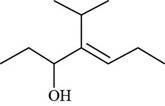
Figure 7
The numbering of carbon atoms present in longest carbon chain of the given compound is shown below.
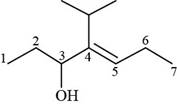
Figure 8
The longest carbon chain has seven carbon atoms. The root word used for seven carbon atoms is hept and the suffix used for
The IUPAC name for the given compound is
(e)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 10.38P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
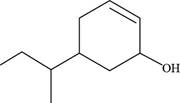
Figure 9
The numbering of carbon atoms present in longest carbon chain of the given compound is shown below.
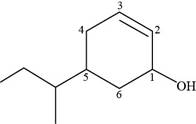
Figure 10
The longest carbon chain has six carbon atoms. The root word used for six carbon atoms is hex and the suffix used for
The IUPAC name of the given structure is
(f)
Interpretation: The IUPAC name for the given compound is to be stated.
Concept introduction: The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from structural formula are
1. First identify the longest carbon chain.
2. The next step is to identify the groups attached to the longest chain.
3. Identify the position, location, and number of the substituent bonded to the carbon chain.
4. Use prefix di, tri, tetra if same type of substituent is present.
5. Name the substituents in alphabetical order.
Answer to Problem 10.38P
The IUPAC name for the given compound is
Explanation of Solution
The given compound is,
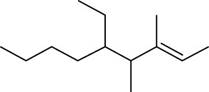
Figure 11
The numbering of carbon atoms present in longest carbon chain of the given compound is shown below.
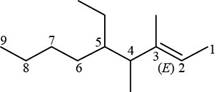
Figure 12
The longest carbon chain has six carbon atoms. The root word used for six carbon atoms is hex and the suffix used for
The IUPAC name for the given compound is
Want to see more full solutions like this?
Chapter 10 Solutions
Organic Chemistry
Additional Science Textbook Solutions
MARINE BIOLOGY
The Cosmic Perspective (8th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
Microbiology with Diseases by Body System (5th Edition)
General, Organic, and Biological Chemistry - 4th edition
- 1. (4 pts-2 pts each part) Consider the crystal structures of NaCl, ZnS, and CsCl (not necessarily shown in this order). a. For one of the three compounds, justify that the unit cell is consistent with stoichiometry of the compound. b. In each of the crystal structures, the cations reside in certain holes in the anions' packing structures. For each compound, what type of holes are occupied by the cations and explain why those particular types of holes are preferred.arrow_forward(2 pts) What do you expect to happen in a Na2O crystal if a Cl− ion replaces one of the O2−ions in the lattice?arrow_forward(2 pts) WSe2 is an ionic compound semiconductor that can be made to be p-type or n-type.What must happen to the chemical composition for it to be p-type? What must happen tothe chemical composition for it to be n-type?arrow_forward
- 8. (2 pts) Silicon semiconductors have a bandgap of 1.11 eV. What is the longest photon wavelength that can promote an electron from the valence band to the conduction band in a silicon-based photovoltaic solar cell? Show all work. E = hv = hc/λ h = 6.626 x 10-34 Js c = 3.00 x 108 m/s 1 eV 1.602 x 10-19 Jarrow_forwardA solution containing 100.0 mL of 0.155 M EDTA buffered to pH 10.00 was titrated with 100.0 mL of 0.0152 M Hg(ClO4)2 in a cell: calomel electrode (saturated)//titration solution/Hg(l) Given the formation constant of Hg(EDTA)2-, logKf= 21.5, and alphaY4-=0.30, find out the cell voltage E. Hg2+(aq) + 2e- = Hg(l) E0= 0.852 V E' (calomel electrode, saturated KCl) = 0.241 Varrow_forwardFrom the following reduction potentials I2 (s) + 2e- = 2I- (aq) E0= 0.535 V I2 (aq) + 2e- = 2I- (aq) E0= 0.620 V I3- (aq) + 2e- = 3I- (aq) E0= 0.535 V a) Calculate the equilibrium constant for I2 (aq) + I- (aq) = I3- (aq). b) Calculate the equilibrium constant for I2 (s) + I- (aq) = I3- (aq). c) Calculate the solubility of I2 (s) in water.arrow_forward
- 2. (3 pts) Consider the unit cell for the spinel compound, CrFe204. How many total particles are in the unit cell? Also, show how the number of particles and their positions are consistent with the CrFe204 stoichiometry - this may or may not be reflected by the particle colors in the diagram. (HINT: In the diagram, the blue particle is in an interior position while each red particle is either in a corner or face position.)arrow_forwardFrom the following potentials, calculate the activity of Cl- in saturated KCl. E0 (calomel electrode)= 0.268 V E (calomel electrode, saturated KCl)= 0.241 Varrow_forwardCalculate the voltage of each of the following cells. a) Fe(s)/Fe2+ (1.55 x 10-2 M)//Cu2+ (6.55 x 10-3 M)/Cu(s) b) Pt, H2 (0.255 bar)/HCl (4.55 x 10-4 M), AgCl (sat'd)/Ag Fe2+ +2e- = Fe E0= -0.44 V Cu2+ + 2e- = Cu E0= 0.337 V Ag+ + e- = Ag E0= 0.799 V AgCl(s) + e- = Ag(s) + Cl- E0= 0.222 V 2H+ + 2e- = H2 E0= 0.000 Varrow_forward
- A solution contains 0.097 M Ce3+, 1.55x10-3 M Ce4+, 1.55x10-3 M Mn2+, 0.097 M MnO4-, and 1.00 M HClO4 (F= 9.649 x 104 C/mol). a) Write a balanced net reaction that can occur between species in this solution. b) Calculate deltaG0 and K for the reaction. c) Calculate E and deltaG for the conditions given. Ce4+ + e- = Ce3+ E0= 1.70 V MnO4- + 8H+ + 5e- = Mn2+ + 4H2O E0= 1.507 Varrow_forward1. Provide a step-by-step mechanism for formation of ALL STEREOISOMERS in the following reaction. Na HCO3 (Sodium bicarbonate, baking soda) is not soluble in CH2Cl2. The powder is a weak base used to neutralize strong acid (pKa < 0) produced by the reaction. Redraw the product to show the configuration(s) that form at C-2 and C-4. Br2 OH CH2Cl2 Na* HCO3 Br HO OH + Na Br +arrow_forward2. Specify the solvent and reagent(s) required to carry out each of the following FGI. If two reagent sets must be used for the FGI, specify the solvent and reagent(s) for each reagent set. If a reaction cannot be carried out with reagents (sets) class, write NP (not possible) in the solvent box for reagent set #1. Use the letter abbreviation for each solvent; use a number abbreviation for reagent(s). Solvents: CH2Cl2 (A); H₂O (B); Reagents: HBr (1); R₂BH (6); H2SO4 (2); CH3OH (C); Br₂ (3); CH3CO₂H (D) NaHCO3 (4); Hg(OAc)2 (5); H₂O2/HO (7); NaBH4 (8) Reagent Set #1 Reagent Set #2 FGI + enant OH Solvent Reagent(s) Solvent Reagent(s)arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning



