
Consider a N2 molecule in its first excited electronic state, that is, when an electron in the highest occupied molecular orbital is promoted to the lowest empty molecular orbital. (a) Identify the molecular orbitals involved and sketch a diagram to show the transition. (b) Compare the bond order and bond length of N2* with N2, where the asterisk denotes the excited molecule. (c) Is N2* diamagnetic or paramagnetic? (d) When N2* loses its excess energy and converts to the ground state N2, it emits a photon of wavelength 470 nm, which makes up part of the auroras lights. Calculate the energy difference between these levels.
(a)
Interpretation:
The molecular orbital involved in transition should be identified and to sketch the transition. Bond order of 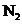 and
and 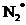 should be found and the bond length should be compared. The magnetic properties of
should be found and the bond length should be compared. The magnetic properties of 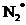 should be found out. The energy difference of the given transition should be determined
should be found out. The energy difference of the given transition should be determined
Concept Introduction:
- In molecular orbital theory, when the bonding takes place the atomic orbitals that take part combine to get a new orbital that has the properties of the whole molecule. The newly formed orbitals are known as molecular orbitals
- The bond order gives an idea about the stability of a molecule. It can be calculated using the molecular orbital theory. The stability of a molecule increase as the bond order increases.
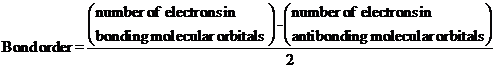
- Paramagnetic species contains at least one unpaired electrons and can be attracted towards magnetic fields. Diamagnetic species does have any unpaired electrons. That is spins of all the electrons are paired. It slightly repelled towards the magnetic fields
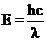

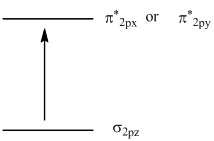
To identify: molecular orbital involved in transition and to sketch the transition.
Answer to Problem 10.113QP
The transition sketch is,
Explanation of Solution
In molecular orbital theory, when the bonding takes place the atomic orbitals that take part combine to get a new orbital that has the properties of the whole molecule. The newly formed orbitals are known as molecular orbitals and only contain a maximum of two electrons. The number of newly formed molecular orbital is equal to the number of atomic orbitals involved in the bonding.
There are two types of molecular orbitals,
- a) Bonding molecular orbitals: sharing of electron density is between the nuclei and has comparatively lower energy and fills first.
- b) Antibonding molecular orbitals: Two nuclei is pulled by the electrons density in opposite direction and has higher energy comparing to bonding molecular orbital.
Molecular orbital diagram of  is given below
is given below
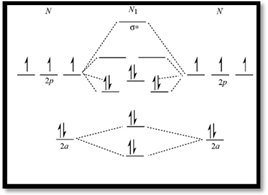
Figure 1
In the ground state of 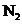 the electrons are in
the electrons are in 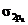 orbital when the
orbital when the 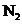 gets excited by getting energy the electron move to
gets excited by getting energy the electron move to 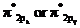 orbitals.
orbitals.
The diagram that showing transition is given below,

(b)
Interpretation:
The molecular orbital involved in transition should be identified and to sketch the transition. Bond order of 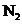 and
and 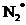 should be found and the bond length should be compared. The magnetic properties of
should be found and the bond length should be compared. The magnetic properties of 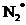 should be found out. The energy difference of the given transition should be determined
should be found out. The energy difference of the given transition should be determined
Concept Introduction:
- In molecular orbital theory, when the bonding takes place the atomic orbitals that take part combine to get a new orbital that has the properties of the whole molecule. The newly formed orbitals are known as molecular orbitals
- The bond order gives an idea about the stability of a molecule. It can be calculated using the molecular orbital theory. The stability of a molecule increase as the bond order increases.
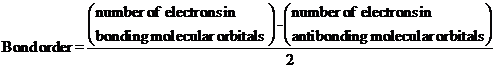
- Paramagnetic species contains at least one unpaired electrons and can be attracted towards magnetic fields. Diamagnetic species does have any unpaired electrons. That is spins of all the electrons are paired. It slightly repelled towards the magnetic fields
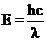

To identify: Bond of order of 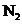 and
and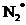 . Also to compare its bond length
. Also to compare its bond length
Answer to Problem 10.113QP
Bond order of 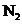 and
and 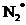 is 3 and 2 respectively. Also the bond length of
is 3 and 2 respectively. Also the bond length of 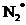 is longer than
is longer than 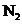 .
.
Explanation of Solution
Electronic configuration of excited nitrogen molecule 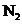 is
is 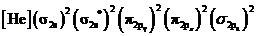
The bond order gives an idea about the stability of a molecule. It can be calculated using the molecular orbital theory. The stability of a molecule increase as the bond order increases.
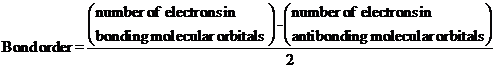
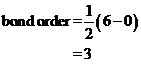
Electronic configuration of excited nitrogen molecule 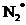 is
is 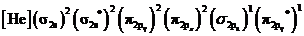
The bond order gives an idea about the stability of a molecule. It can be calculated using the molecular orbital theory. The stability of a molecule increase as the bond order increases.
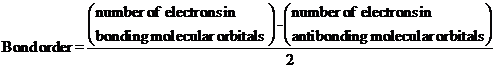
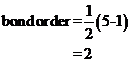
Bond order of  is 3 whereas
is 3 whereas 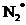 is 2.
is 2.
Therefore, the bond length of 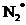 is longer than
is longer than 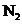 .
.
(c)
Interpretation:
The molecular orbital involved in transition should be identified and to sketch the transition. Bond order of 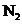 and
and 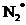 should be found and the bond length should be compared. The magnetic properties of
should be found and the bond length should be compared. The magnetic properties of 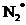 should be found out. The energy difference of the given transition should be determined
should be found out. The energy difference of the given transition should be determined
Concept Introduction:
- In molecular orbital theory, when the bonding takes place the atomic orbitals that take part combine to get a new orbital that has the properties of the whole molecule. The newly formed orbitals are known as molecular orbitals
- The bond order gives an idea about the stability of a molecule. It can be calculated using the molecular orbital theory. The stability of a molecule increase as the bond order increases.
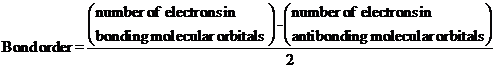
- Paramagnetic species contains at least one unpaired electrons and can be attracted towards magnetic fields. Diamagnetic species does have any unpaired electrons. That is spins of all the electrons are paired. It slightly repelled towards the magnetic fields
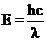

To identify: The magnetic properties of 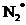
Answer to Problem 10.113QP
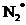 is diamagnetic
is diamagnetic
Explanation of Solution
Paramagnetic species contains at least one unpaired electrons and can be attracted towards magnetic fields. Diamagnetic species does have any unpaired electrons. That is spins of all the electrons are paired. It slightly repelled towards the magnetic fields.
Electronic configuration of excited nitrogen molecule 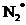 is
is 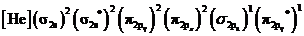
Even though there are unpaired electrons, the spin of the electrons was not change in the time of transition. All the electrons are paired so it is diamagnetic.
(d)
Interpretation:
The molecular orbital involved in transition should be identified and to sketch the transition. Bond order of 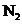 and
and 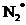 should be found and the bond length should be compared. The magnetic properties of
should be found and the bond length should be compared. The magnetic properties of 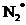 should be found out. The energy difference of the given transition should be determined
should be found out. The energy difference of the given transition should be determined
Concept Introduction:
- In molecular orbital theory, when the bonding takes place the atomic orbitals that take part combine to get a new orbital that has the properties of the whole molecule. The newly formed orbitals are known as molecular orbitals
- The bond order gives an idea about the stability of a molecule. It can be calculated using the molecular orbital theory. The stability of a molecule increase as the bond order increases.
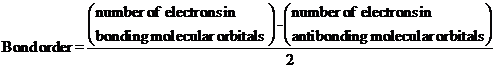
- Paramagnetic species contains at least one unpaired electrons and can be attracted towards magnetic fields. Diamagnetic species does have any unpaired electrons. That is spins of all the electrons are paired. It slightly repelled towards the magnetic fields
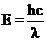

To determine: The energy difference of the given transition.
Answer to Problem 10.113QP
The energy difference of the given transition is 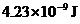
Explanation of Solution
The energy of light is calculated below.
Given,
The wavelength of light is 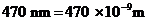 .
.
Planck’s constant is 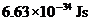
Speed of the light is 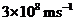
The energy of light is calculated is calculated by the equation,
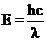

Substituting the given values in the equation,
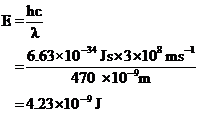
The energy difference of the given transition is
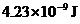
Want to see more full solutions like this?
Chapter 10 Solutions
EBK CHEMISTRY
- esc 2 Incorrect Feedback: Your answer is incorrect. Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? A O • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. . If your answer is no, check the box under the drawing area instead. Check F1 ! @ X C Save For Later Submit Assignment 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 80 et A ད 1 4 F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 # $ 45 % A 6 87 & * 8 9 ) 0 + ||arrow_forwardCan the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ?A Δ O • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilit ku F11arrow_forward१ eq ine teaching and × + rn/takeAssignment/takeCovalentActivity.do?locator-assignment-take [Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is In progress mit Answer Retry Entire Group 5 more group attempts remaining Cengage Learning | Cengage Technical Support Save and Exitarrow_forward
- Draw the molecules.arrow_forwardDraw the mechanism for the acid-catalyzed dehydration of 2-methyl-hexan-2-ol with arrows please.arrow_forward. Draw the products for addition reactions (label as major or minor) of the reaction between 2-methyl-2-butene and with following reactants : Steps to follow : A. These are addition reactions you need to break a double bond and make two products if possible. B. As of Markovnikov rule the hydrogen should go to that double bond carbon which has more hydrogen to make stable products or major product. Here is the link for additional help : https://study.com/academy/answer/predict-the-major-and-minor-products-of-2-methyl- 2-butene-with-hbr-as-an-electrophilic-addition-reaction-include-the-intermediate- reactions.html H₂C CH3 H H3C CH3 2-methyl-2-butene CH3 Same structure CH3 IENCESarrow_forward
- Draw everything on a piece of paper including every single step and each name provided using carbons less than 3 please.arrow_forwardTopics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. H The IUPAC name isarrow_forward[Review Topics] [References] Write an acceptable IUPAC name for the compound below. (Only systematic names, not common names are accepted by this question.) Keep the information page open for feedback reference. The IUPAC name is Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forward
- Please draw.arrow_forwardA chromatogram with ideal Gaussian bands has tR = 9.0 minutes and w1/2 = 2.0 minutes. Find the number of theoretical plates that are present, and calculate the height of each theoretical plate if the column is 10 centimeters long.arrow_forwardAn open tubular column has an inner diameter of 207 micrometers, and the thickness of the stationary phase on the inner wall is 0.50 micrometers. Unretained solute passes through in 63 seconds and a particular solute emerges at 433 seconds. Find the distribution constant for this solute and find the fraction of time spent in the stationary phase.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
