
Chemistry in Context
8th Edition
ISBN: 9780073522975
Author: American Chemical Society
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 53Q
Here are two scanning electron micrograph images of particulate matter, courtesy of the National Science Foundation and researchers at Arizona State University. The first is of a soil particle and the second of a rubber particle, and each is about 10 μm in diameter.
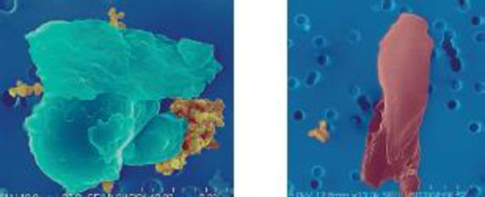
(both) Source: National Science Foundation, Courtesy of Xin Hua & James Anderson
- a. Suggest a likely source of the rubber particle. Name two other substances that might contribute PM to the air.
- b. The soil particle is composed mainly of silicon and oxygen. What other elements are commonly present in the rocks and minerals in Earth’s crust?
- c. What about these photographs suggests that these particles would inflame your blood vessels?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Q14. Fill this chart: (please refer to ppt notes/browser to answer these questions)
What alcohol is also called wood alcohol?
What is the common name of ethanol?
Draw the structure of phenol and thiophene?
Are bigger chain alcohol like heptanol and octanol
are soluble or insoluble in water and explain it ?
Are ethers soluble or insoluble in water?
What suffix and prefix are used for alcohol while
naming alcohol and ether?
What the process called when we add water to any
alkene to make alcohol?
Q16. Draw the diagram of following aromatic compound (practice from previous module)
Aniline
Phenol
Benzoic acid
Methyl benzoate
Q17. a. Write the oxidation reactions for the 2 propanol.
b. Write the oxidation reaction of the ethanol.
Question 11 of 18 (1 point) Question Attempt: 3 of
How many signals do you expect in the 'H NMR spectrum for this molecule?
Br
Br
Write the answer below.
Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H
atoms that would contribute to the same signal as the H already highlighted red.
Note for advanced students: In this question, any multiplet is counted as one signal.
Number of signals in the 'H NMR spectrum.
1
For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to
the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box at right.
No additional Hs to color in top
molecule
Check
For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute
to the same signal as the H atom already highlighted red.
If no other H atoms will contribute, check the box…
Organic Chemistry
Esterification reactions
1. Write the steps to prepare ester.
2. Write complete reaction of ethanol and acetic acid to make ester.
3. What does ester smell like? What are the uses of ester.
4. What the role of sulfuric acid in the esterification reaction
Chapter 1 Solutions
Chemistry in Context
Ch. 1.1 - Prob. 1.2CTCh. 1.1 - The air is different in a pine forest, a bakery,...Ch. 1.1 - Scientific Practices More Oxygen ? We live in an...Ch. 1.2 - Prob. 1.5SCCh. 1.3 - Sulfur dioxide (SO2) is released in the air when...Ch. 1.4 - Prob. 1.9YTCh. 1.5 - Prob. 1.10CTCh. 1.6 - Prob. 1.11YTCh. 1.11 - Prob. 1.19YTCh. 1.11 - Prob. 1.21YT
Ch. 1.12 - Summarize what you have learned about ozone...Ch. 1.13 - Prob. 1.27YTCh. 1.13 - Prob. 1.28YTCh. 1.13 - Prob. 1.29SCCh. 1.13 - Prob. 1.30YTCh. 1.13 - Prob. 1.35CTCh. 1 - Prob. 1QCh. 1 - Prob. 2QCh. 1 - Prob. 3QCh. 1 - Identify three sources of particulate matter found...Ch. 1 - Prob. 5QCh. 1 - Prob. 6QCh. 1 - In these diagrams, two different types of atoms...Ch. 1 - Prob. 8QCh. 1 - Prob. 9QCh. 1 - Prob. 10QCh. 1 - Prob. 11QCh. 1 - Prob. 12QCh. 1 - Prob. 13QCh. 1 - Consider the following blank periodic table. a....Ch. 1 - Classify each of these substances as an element, a...Ch. 1 - Prob. 16QCh. 1 - Hydrocarbons are important fuels that we burn...Ch. 1 - Prob. 18QCh. 1 - Arrange these types of radiation in order of...Ch. 1 - These questions relate to the combustion of...Ch. 1 - Balance these equations in which ethane (C2 H4)...Ch. 1 - Prob. 22QCh. 1 - Count the atoms on both sides of the equation to...Ch. 1 - Prob. 24QCh. 1 - Nail polish remover containing acetone was spilled...Ch. 1 - Prob. 26QCh. 1 - Prob. 27QCh. 1 - Prob. 28QCh. 1 - Prob. 29QCh. 1 - Prob. 30QCh. 1 - A headline from the Anchorage Daily News in Alaska...Ch. 1 - Prob. 32QCh. 1 - Consider how life on Earth would change if the...Ch. 1 - Prob. 34QCh. 1 - Prob. 35QCh. 1 - Prob. 36QCh. 1 - Prob. 37QCh. 1 - Prob. 38QCh. 1 - Prob. 39QCh. 1 - Prob. 40QCh. 1 - Prob. 41QCh. 1 - Prob. 42QCh. 1 - Prob. 43QCh. 1 - Prob. 44QCh. 1 - Prob. 45QCh. 1 - Prob. 46QCh. 1 - Prob. 47QCh. 1 - Prob. 48QCh. 1 - Prob. 49QCh. 1 - Mercury, another serious air pollutant, is not...Ch. 1 - The EPA oversees the Presidential Green Chemistry...Ch. 1 - Prob. 52QCh. 1 - Here are two scanning electron micrograph images...Ch. 1 - Prob. 54QCh. 1 - Prob. 55QCh. 1 - Prob. 56QCh. 1 - Prob. 57QCh. 1 - You may have admired the beauty of hardwood...Ch. 1 - Prob. 59Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
What are the cervical and lumbar enlargements?
Principles of Anatomy and Physiology
1. Rub your hands together vigorously. What happens? Discuss the energy transfers and transformations that take...
College Physics: A Strategic Approach (3rd Edition)
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
Why is it unlikely that two neighboring water molecules would be arranged like this?
Campbell Biology (11th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 11. Complete the following esterification reaction with names of all the reactants and products under. Hint: Remove the water and end up with ester R-C-OH + ROH R-C-OR + H₂O A carboxylic acid An alcohol An ester Water BYJU'S H-C-C O-H Нин C-C-C-H HAAA H O-C-C-C-H AAA Ethanoic acid Propanol Water Propyl ethanoate By com CH3COOH + CH3CH2CH2CH₂CH₂OH → Practice for alcohols aldehydes and ketones: 12. Draw the structures from the following names mixed of alcohol/aldehyde and ketone: a. 4-methyl cyclohexanone b. 3-methyl-2-pentenal c. 2,3-dimethylcyclohexanone d. 1,3propanediol or Propane 1,3 diol 13. Write systematic names for the following compounds identify functional group: a. b. (CH3)2CH-C OH c) CH(CH₂)-- OH -,-,arrow_forwardmay you please show all steps! i am having a hard time understanding and applying in this format, thank you!arrow_forward10. Complete the substitution reaction of 2 pentanol with these reagents. Reagents & Reaction Conditions use practice sheet. Please write only major products, minor product like water, other gases are not required. Hint: In substitution of alcohol, we generally substitute OH group with Halogens like cl, Br, F using some reagent containing halogens. Ensure to add halogens to the same carbon number where you are removing OH from Examples Alcohols can be converted to Alkyl Halides with HX acids HBr H₂O HCI + H₂O HI + H₂O CH,CH₂OH + SOCI₂ CH,CH₂OH + PCI₁₂ A BBYJU'S CH CHCI + SO₂+ HCI CH₂CH CIP(OH), + HCI CH,CH₂OH + PCI CHCHCI + POCI + HCI CH,CH₂OH + PBr, CH,CH,Br + P(OH), + HBr 1. Reaction with HBr with 2 Pentanol 2.Reaction with HI with 2 pentanol © Byjus.com 3.Reaction with HCI+ZnCl,, with 2 pentanol (Zncl2 is catalyst no role) 4.Reaction with SOCI,, with 2 Pentanol 5.Reaction with PBr; or PCl, with 2 pentanolarrow_forward
- 3. Is 2-methyl-2-propanol a primary, secondary, or tertiary alcohol? Write out the structures of 2-methyl-2-propanol and also any oxidation products of 2- methyl-2- propanol. If there is more than one oxidation product, give the structure of each of the products. 4. 2-Propanol is the IUPAC systematic name of this alcohol. It has a common name by which it is much better known (You'll see it in the grocery store or pharmacy). Give that common name 5. Aldehydes can be synthesized by the oxidation of. Please choose from below choices A. Primary alcohols B. Secondary alcohols C. Organic acids D. Inorganic acids 6. Tertiary alcohol Can undergo oxidation. yes or no. ? If yes then answer the product.arrow_forwardFinish the reactions hand written pleasearrow_forwardPart A Identify each alcohol as primary, secondary, or tertiary Drag the appropriate items to their respective bins. CH₂ H₂C- -C-OH HO CH₂ Primary Он OH CH₂ OH CCH₂OH CH₂ сн Secondary Tertiary Reset Help CH,CH₂ (CH)CHCH,OH CH,CH,CH,CCH, CHOH CH₂ Different types of alcohol groups Alcohol and its reaction: 8. Combing two alcohol molecules below and completing the reaction with Product .( Hint Reaction called etherification as ether is formed and name the ether once you complete the reaction. Hint.: R-O-H+H-O-RR-O-R Do the reaction: CH₂OH + CH₂OH---→ + H-O-H 9. Write the reaction of formation of alcohol from alkene by adding water: Addition reaction also called hydration reaction as we are adding water which occur always in presence of acid Hint: Break the double bond and add H and OH if symmetrical then add anywhere if unsymmetrical then follow Markovnikov rule H should go to that double bone carbon which has more hydrogen CH2=CH2 + H₂O-→arrow_forward
- Complete the reaction hand written pleasearrow_forwardPredict the major products of this organic reaction: HBr (1 equiv) cold ? Some important notes: • Draw the major product, or products, of this reaction in the drawing area below. • You can draw the products in any arrangement you like. • Pay careful attention to the reaction conditions, and only include the major products. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. • Note that there is only 1 equivalent of HBr reactant, so you need not consider the case of multiple additions. dm Re Explanation Check ©2025 McGraw Hill LLC. All Rights Reserved. Termarrow_forwardb) Use curved arrows to show the reaction of the radical with hydrogen bromide. Br: Br H .. Answer Bankarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning
Types of Matter: Elements, Compounds and Mixtures; Author: Professor Dave Explains;https://www.youtube.com/watch?v=dggHWvFJ8Xs;License: Standard YouTube License, CC-BY