
Concept explainers
(a)
Interpretation:Condensed formula that shows multiple bonds, lone pairs should be drawn for the pair
Concept introduction: There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge. The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance, the propane is written in this notation as follows:
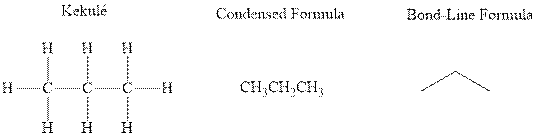
(b)
Interpretation: Condensed formula that shows multiple bonds, lone pairs should be drawn for the pair
Concept introduction: There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge. The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance the propane is written in this notation as follows:
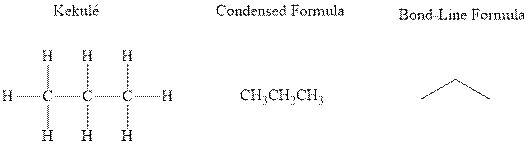
(c)
Interpretation: Condensed formula that shows multiple bonds, lone pairs should be drawn for the pair
Concept introduction: There are three ways of representation of molecular structures. The hashed and wedged formulas have three types of bonds. The bond above the plane is represented by bold hash while bonds behind the plane are represented by wedge. The bonds in plane are represented by normal bonds.
Bond-line formula depicts the zig-zag framework of carbon chain with attached substituents. The terminal position represents a methyl while the non-terminal is for methylene group. For instance the propane is written in this notation as follows:
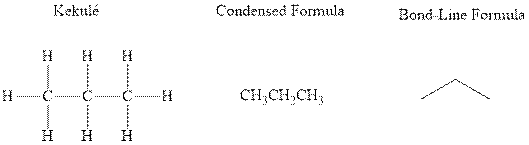
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
EBK STUDY GUIDE/SOLUTIONS MANUAL FOR OR
- =Naming benzene derivatives Name these organic compounds: structure C1 CH3 name ☐ CH3 ப C1 × ☐arrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.arrow_forwardI dont understand this.arrow_forward
- Can you please explain this prooblem to me, show me how the conjugation is added, did I add them in the correct places and if so please show me. Thanks!arrow_forwardBasic strength of organic bases.arrow_forwardNucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See imagearrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





