
Organic Chemistry, Loose-leaf Version
8th Edition
ISBN: 9781305865549
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.73AP
Following is a structural formula of benzene, C6H6, which we study in Chapter 21.
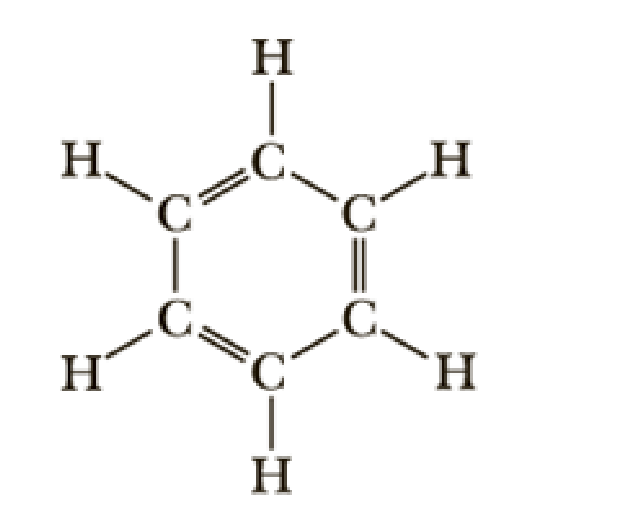
- (a) Using VSEPR, predict each H—C—C and C—C—C bond angle in benzene.
- (b) State the hybridization of each carbon in benzene.
- (c) Predict the shape of a benzene molecule.
- (d) Draw important resonance contributing structures.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Show reaction mechanism. Don't give Ai generated solution
Describe some isomerism that carboranes have.
Indicate an isomerism that carboranes present.
Chapter 1 Solutions
Organic Chemistry, Loose-leaf Version
Ch. 1.1 - Prob. 1.1PCh. 1.2 - Prob. 1.2PCh. 1.2 - Judging from their relative positions in the...Ch. 1.2 - Classify each bond as nonpolar covalent or polar...Ch. 1.2 - Using the symbols and +, indicate the direction...Ch. 1.2 - Draw Lewis structures showing all valence...Ch. 1.2 - Draw Lewis structures for these ions and show...Ch. 1.3 - Draw Lewis structures and condensed structural...Ch. 1.3 - Prob. 1.9PCh. 1.3 - Prob. 1.10P
Ch. 1.3 - Prob. 1.11PCh. 1.3 - Prob. 1.12PCh. 1.4 - Predict all bond angles for these molecules. (a)...Ch. 1.5 - The geometry of carbon in diamond is tetrahedral,...Ch. 1.5 - Because of their spherical shape, C60 molecules...Ch. 1.5 - What best describes the CCC bond angles in C60? 1....Ch. 1.5 - Prob. 1.14PCh. 1.7 - Describe the bonding in these molecules in terms...Ch. 1.8 - Prob. 1.16PCh. 1.8 - Prob. 1.17PCh. 1.8 - Prob. 1.18PCh. 1.9 - Draw three contributing structures of the...Ch. 1.9 - What is the hybridization state of the circled...Ch. 1.9 - The molecule shown on the right in the example in...Ch. 1.9 - Prob. CQCh. 1.9 - The following structure is called imidazolium....Ch. 1 - Write the ground-state electron configuration for...Ch. 1 - Identify the atom that has each ground-state...Ch. 1 - Define valence shell and valence electron.Ch. 1 - How many electrons are in the valence shell of...Ch. 1 - Prob. 1.24PCh. 1 - Prob. 1.25PCh. 1 - Prob. 1.26PCh. 1 - Write Lewis structures for these compounds. Show...Ch. 1 - Write Lewis structures for these ions. Show all...Ch. 1 - Prob. 1.29PCh. 1 - Some of these structural formulas are incorrect...Ch. 1 - Following the rule that each atom of carbon,...Ch. 1 - Following are several Lewis structures showing all...Ch. 1 - Which statements are true about electronegativity?...Ch. 1 - Why does fluorine, the element in the upper right...Ch. 1 - Arrange the single covalent bonds within each set...Ch. 1 - Using the values of electronegativity given in...Ch. 1 - Prob. 1.37PCh. 1 - Use VSEPR to predict bond angles about each...Ch. 1 - Use VSEPR to predict bond angles about each atom...Ch. 1 - Use VSEPR to predict the geometry of these ions....Ch. 1 - Prob. 1.41PCh. 1 - Prob. 1.42PCh. 1 - What is the meaning of the term tertiary (3) when...Ch. 1 - What is the meaning of the term tertiary (3) when...Ch. 1 - Draw structural formulas for (a) The four primary...Ch. 1 - Draw structural formulas for the three tertiary...Ch. 1 - Prob. 1.47PCh. 1 - Identify the functional groups in each compound.Ch. 1 - Draw a three-dimensional representation for each...Ch. 1 - Tetrafluoroethylene, C2F4, is the starting...Ch. 1 - Which statements are true about resonance...Ch. 1 - Prob. 1.52PCh. 1 - Prob. 1.53PCh. 1 - Prob. 1.54PCh. 1 - Are the structures in each set valid contributing...Ch. 1 - State the orbital hybridization of each...Ch. 1 - Describe each highlighted bond in terms of the...Ch. 1 - Following is a structural formula of the...Ch. 1 - Draw a Lewis structure for methyl isocyanate,...Ch. 1 - What is the hybridization of the highlighted atoms...Ch. 1 - Using cartoon representations, draw a molecular...Ch. 1 - In what kind of orbitals do the lone-pair...Ch. 1 - Draw the delocalized molecular orbitals for the...Ch. 1 - Prob. 1.64APCh. 1 - Each compound contains both ions and covalent...Ch. 1 - Predict whether the carbon-metal bond in these...Ch. 1 - Prob. 1.67APCh. 1 - Phosphorus is immediately under nitrogen in the...Ch. 1 - Draw a Lewis structure for the azide ion, N3. (The...Ch. 1 - Cyanic acid, HOCN, and isocyanic acid, HNCO,...Ch. 1 - In Chapter 6, we study a group of organic cations...Ch. 1 - Many reactions involve a change in hybridization...Ch. 1 - Following is a structural formula of benzene,...Ch. 1 - Following are three contributing structures for...Ch. 1 - (a) Draw a Lewis structure for the ozone molecule,...Ch. 1 - The following two compounds are isomers; that is,...Ch. 1 - In future chapters, we will encounter...Ch. 1 - Prob. 1.78AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Transmitance 3. Which one of the following compounds corresponds to this IR spectrum? Point out the absorption band(s) that helped you decide. OH H3C OH H₂C CH3 H3C CH3 H3C INFRARED SPECTRUM 0.8- 0.6 0.4- 0.2 3000 2000 1000 Wavenumber (cm-1) 4. Consider this compound: H3C On the structure above, label the different types of H's as A, B, C, etc. In table form, list the labeled signals, and for each one state the number of hydrogens, their shifts, and the splitting you would observe for these hydrogens in the ¹H NMR spectrum. Label # of hydrogens splitting Shift (2)arrow_forwardNonearrow_forwardDraw the Lewis structure of C2H4Oarrow_forward
- a) 5. Circle all acidic (and anticoplanar to the Leaving group) protons in the following molecules, Solve these elimination reactions, and identify the major and minor products where appropriate: 20 points + NaOCH3 Br (2 productarrow_forwardNonearrow_forwardDr. Mendel asked his BIOL 260 class what their height was and what their parent's heights were. He plotted that data in the graph below to determine if height was a heritable trait. A. Is height a heritable trait? If yes, what is the heritability value? (2 pts) B. If the phenotypic variation is 30, what is the variation due to additive alleles? (2 pts) Offspring Height (Inches) 75 67.5 60 52.5 y = 0.9264x + 4.8519 55 60 65 MidParent Height (Inches) 70 75 12pt v V Paragraph B IUA > AT2 v Varrow_forward
- Experiment: Each team will be provided with 5g of a mixture of acetanilide and salicylic acid. You will divide it into three 1.5 g portions in separate 125 mL Erlenmeyer flasks savıng some for melting point analysis. Dissolve the mixture in each flask in ~60mL of DI water by heating to boiling on a hotplate. Take the flasks off the hotplate once you have a clear solution and let them stand on the bench top for 5 mins and then allow them to cool as described below. Sample A-Let the first sample cool slowly to room temperature by letting it stand on your lab bench, with occasional stirring to promote crystallization. Sample B-Cool the second sample 1n a tap-water bath to 10-15 °C Sample C-Cool the third sample in an ice-bath to 0-2 °C Results: weight after recrystalization and melting point temp. A=0.624g,102-115° B=0.765g, 80-105° C=1.135g, 77-108 What is the percent yield of A,B, and C.arrow_forwardRel. Intensity Q 1. Which one of the following is true of the compound whose mass spectrum is shown here? Explain how you decided. 100 a) It contains chlorine. b) It contains bromine. c) It contains neither chlorine nor bromine. 80- 60- 40- 20- 0.0 0.0 TT 40 80 120 160 m/z 2. Using the Table of IR Absorptions how could you distinguish between these two compounds in the IR? What absorbance would one compound have that the other compound does not? HO CIarrow_forwardIllustrate reaction mechanisms of alkenes with water in the presence of H2SO4, detailing each step of the process. Please show steps of processing. Please do both, I will thumb up for sure #1 #3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY