
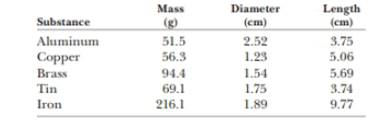
The data in the following table represent measurements of the masses and dimensions of solid cylinders of aluminum, copper, brass, tin, and iron. (a) Use these data to calculate the densities of these substances. (b) State how your results compare with those given in Table 14.1.
(a)
The densities of each substance.
Answer to Problem 1.61AP
The density of aluminum solid cylinders is 2.75 g/cm3, density of copper solid cylinders is 9.36 g/cm3, brass solid cylinders is 8.91 g/cm3, tin solid cylinders is 7.68 g/cm3 and iron solid cylinders is 7.88 g/cm3.
Explanation of Solution
Given info: The mass, diameter and length of each substance are given below,
| Substance | Mass (g) | Diameter (cm) | Length (cm) |
| Aluminum | 51.5 | 2.52 | 3.75 |
| Copper | 56.3 | 1.23 | 5.06 |
| Brass | 94.4 | 1.54 | 5.69 |
| Tin | 69.1 | 1.75 | 3.74 |
| Iron | 216.1 | 1.89 | 9.77 |
Formula to calculate the density of substance is,
ρ=mV (1)
Here,
m is the mass of substance.
V is the volume of substance.
Write the expression for the volume of solid cylinder,
V=π(d2)2l
Here,
d is the diameter of the substance.
l is length of the substance.
Substitute π(d2)2l for V in the equation (1).
ρ=mπ(d2)2l=4mπd2l (2)
For aluminum:
Substitute 51.5 g for m, 2.52 cm for d and 3.75 cm for l in the equation (2).
ρ=4(51.5 g)π(2.52 cm)2(3.75 cm)=2.75 g/cm3
Thus, the density of aluminum solid cylinders is 2.75 g/cm3.
For copper:
Substitute 56.3 g for m, 1.23 cm for d and 5.06 cm for l in the equation (2).
ρ=4(56.3 g)π(1.23 cm)2(5.06 cm)=9.36 g/cm3
Thus, the density of copper solid cylinders is 9.36 g/cm3.
For brass:
Substitute 94.4 g for m, 1.54 cm for d and 5.69 cm for l in the equation (2).
ρ=4(94.4 g)π(1.54 cm)2(5.69 cm)=8.91 g/cm3
Thus, the density of brass solid cylinders is 8.91 g/cm3.
For tin:
Substitute 69.1 g for m, 1.75 cm for d and 3.74 cm for l in the equation (2).
ρ=4(69.1 g)π(1.75 cm)2(3.74 cm)=7.68 g/cm3
Thus, the density of tin solid cylinders is 7.68 g/cm3.
For iron:
Substitute 216.1 g for m, 1.89 cm for d and 9.77 cm for l in the equation (2).
ρ=4(216.1 g)π(1.89 cm)2(9.77 cm)=7.88 g/cm3
Thus, the density of iron solid cylinders is 7.88 g/cm3.
Conclusion:
Therefore, the density of aluminum solid cylinders is 2.75 g/cm3, density of copper solid cylinders is 9.36 g/cm3, brass solid cylinders is 8.91 g/cm3, tin solid cylinders is 7.68 g/cm3 and iron solid cylinders is 7.88 g/cm3.
(b)
The comparison between results of part (a) and table 14.1.
Answer to Problem 1.61AP
The density of aluminum from table is 2% less than the density of aluminum from result of part (a), the density of copper from table is 5% less than the density of copper from result of part (a), the density of brass from table is 6% less than the density of brass from result of part (a), the density of tin from table is 5% less than the density of tin from result of part (a) and the density of iron from table is 0.3% less than the density of iron from result of part (a).
Explanation of Solution
Given info:
Formula to calculate the percentage error is,
percentage error=(ρ−ρ′ρ′)×100 (3)
Here,
ρ is the density of the aluminum from the result.
ρ′ is the density of aluminum from table 14.1.
For aluminum:
From part (a), the density of the aluminum is 2.75 g/cm3 and from table 14.1 the density of aluminum is 2.70 g/cm3.
Substitute 2.75 g/cm3 for ρ and 2.70 g/cm3 for ρ′ in the equation (3).
percentage error=(2.75 g/cm3−2.70 g/cm32.70 g/cm3)×100=1.85%≈2%
Thus, the density of aluminum from table is 2% less than the density of aluminum from result of part (a).
For copper:
From part (a), the density of the copper is 9.37 g/cm3 and from table 14.1 the density of copper is 8.92 g/cm3.
Substitute 9.37 g/cm3 for ρ and 8.92 g/cm3 for ρ′ in the equation (3).
percentage error=(9.37 g/cm3−8.92 g/cm38.92 g/cm3)×100=5%
Thus, the density of copper from table is 5% less than the density of copper from result of part (a).
For brass:
From part (a), the density of the brass is 8.91 g/cm3 and from table 14.1 the density of brass is 8.4 g/cm3.
Substitute 8.91 g/cm3 for ρ and 8.4 g/cm3 for ρ′ in the equation (3).
percentage error=(8.91 g/cm3−8.4 g/cm38.4 g/cm3)×100=6%
Thus, the density of brass from table is 6% less than the density of brass from result of part (a).
For tin:
From part (a), the density of the tin is 7.68 g/cm3 and from table 14.1 the density of tin is 7.30 g/cm3.
Substitute 7.68 g/cm3 for ρ and 7.30 g/cm3 for ρ′ in the equation (3).
percentage error=(7.68 g/cm3−7.30 g/cm37.30 g/cm3)×100=5.2%≈5%
Thus, the density of tin from table is 5% less than the density of tin from result of part (a).
For iron:
From part (a), the density of the iron is 7.88 g/cm3 and from table 14.1 the density of iron is 7.86 g/cm3.
Substitute 7.88 g/cm3 for ρ and 7.86 g/cm3 for ρ′ in the equation (3).
percentage error=(7.88 g/cm3−7.86 g/cm37.86 g/cm3)×100≈0.3%
Thus, the density of iron from table is 3% less than the density of iron from result of part (a).
Conclusion:
Therefore, the density of aluminum from table is 2% less than the density of aluminum from result of part (a), the density of copper from table is 5% less than the density of copper from result of part (a), the density of brass from table is 6% less than the density of brass from result of part (a), the density of tin from table is 5% less than the density of tin from result of part (a) and the density of iron from table is 0.3% less than the density of iron from result of part (a).
Want to see more full solutions like this?
Chapter 1 Solutions
Physics for Scientists and Engineers, Technology Update (No access codes included)
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
Loose Leaf For Integrated Principles Of Zoology
Chemistry & Chemical Reactivity
Biology: Life on Earth (11th Edition)
Fundamentals Of Thermodynamics
Fundamentals of Anatomy & Physiology (11th Edition)
- No chatgpt pls will upvote Already got wrong chatgpt answerarrow_forwardPART III - RESISTORS IN PARALLEL Consider (but do not yet build) the circuit shown in the circuit diagram to the left, which we will call Circuit 3. Make sure you are using Bert bulbs. You may want to wire two batteries in series rather than use a single battery. 7. Predict: a) How will the brightness of bulb B3A compare to the brightness to bulb B3B? c) X E B3A b) How will the brightness of bulb BзA compare to the brightness of bulb B₁ from Circuit 1? How will the currents at points X, Y, and Z be related? www d) How will the current at point X in this circuit compare to the current at point X from Circuit 1? Y Z B3B wwwarrow_forwardPART II - RESISTORS IN SERIES Consider (but do not yet build) the circuit shown in the circuit diagram to the left, which we will call Circuit 2. Make sure you are using Bert bulbs. You may want to wire two batteries in series rather than use a single battery. 4. Predict: a) How will the brightness of bulb B₂ compare to the brighness to bulb B2B? X B2A E Y B2B Ꮓ b) How will the brightness of bulb B2A compare to the brightness of bulb B₁ from Circuit 1? c) How will the currents at points X, Y, and Z be related? d) How will the current at point X in this circuit compare to the current at point X from Circuit 1?arrow_forward
- No chatgpt pls will upvote Already got wrong chatgpt answerarrow_forwardWhat is the practical benefit (in terms of time savings and efficiency) of defining the potential energy? Be clear about what is required in terms of calculation if we do not use the concept of potential energy.arrow_forwardWhat is the critical angle fir the light travelling from the crown glass(n=1.52) into the air(n=1.00)?arrow_forward
- No chatgpt pls will upvotearrow_forwardYou are working with a team that is designing a new roller coaster-type amusement park ride for a major theme park. You are present for the testing of the ride, in which an empty 150 kg car is sent along the entire ride. Near the end of the ride, the car is at near rest at the top of a 100 m tall track. It then enters a final section, rolling down an undulating hill to ground level. The total length of track for this final section from the top to the ground is 250 m. For the first 230 m, a constant friction force of 370 N acts from computer-controlled brakes. For the last 20 m, which is horizontal at ground level, the computer increases the friction force to a value required for the speed to be reduced to zero just as the car arrives at the point on the track at which the passengers exit. (a) Determine the required constant friction force (in N) for the last 20 m for the empty test car. Write AK + AU + AE int = W+Q + TMW + TMT + TET + TER for the car-track-Earth system and solve for…arrow_forward= 12 kg, and m3 Three objects with masses m₁ = 3.8 kg, m₂ find the speed of m3 after it moves down 4.0 m. m/s 19 kg, respectively, are attached by strings over frictionless pulleys as indicated in the figure below. The horizontal surface exerts a force of friction of 30 N on m2. If the system is released from rest, use energy concepts to m m2 m3 iarrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College





