
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
9th Edition
ISBN: 9781292151229
Author: Wade, LeRoy G.
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.46SP
Use resonance structures to identify the areas of high and low electron density in the following compounds.
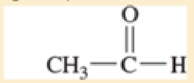
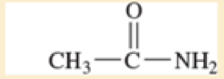
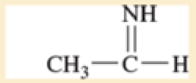
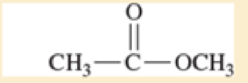
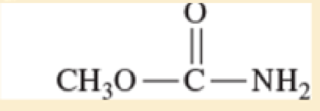
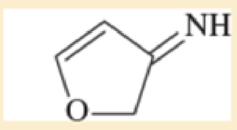
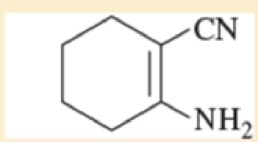
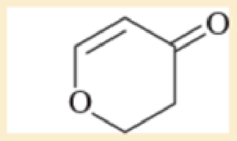
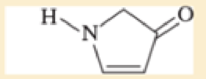
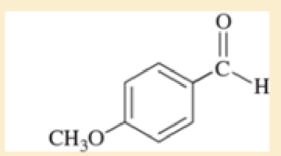
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Basic strength of organic bases.
Nucleophilic Aromatic Substitution: What is the product of the reaction? What is the name of the intermediate complex? *See image
Predict the final product. If 2 products are made, list which should be “major” and “minor” *see attached
Chapter 1 Solutions
Organic Chemistry Plus Masteringchemistry With Pearson Etext, Global Edition
Ch. 1.2C - a. Nitrogen has relatively stable isotopes...Ch. 1.4 - Draw Lewis structures for the following compounds....Ch. 1.5 - Write Lewis structures for the following molecular...Ch. 1.5 - Circle any lone pairs (pairs of nonbonding...Ch. 1.6 - Use electronegativities to predict the direction...Ch. 1.8 - Prob. 1.6PCh. 1.9B - Draw the important resonance forms for the...Ch. 1.9B - Prob. 1.8PCh. 1.9B - Prob. 1.9PCh. 1.9B - Use resonance structures to identify the areas of...
Ch. 1.10A - Draw complete Lewis structures for the following...Ch. 1.10B - Give Lewis structures corresponding to the...Ch. 1.10B - Prob. 1.13PCh. 1.11 - Compute the empirical and molecular formulas for...Ch. 1.16 - a. Use your molecular models to make ethane, and...Ch. 1.17 - a. Predict the hybridization of the oxygen atom in...Ch. 1.17 - Predict the hybridization geometry and bond angles...Ch. 1.17 - Predict the hybridization, geometry, and bond...Ch. 1.17 - Prob. 1.19PCh. 1.17 - Allene, CH2=C=CH2, has the structure shown below...Ch. 1.17 - 1. Draw the important resonance forms for each...Ch. 1.18B - Prob. 1.22PCh. 1.18B - Two compounds with the formula CH3CH=NCH3 are...Ch. 1.19B - Prob. 1.24PCh. 1.19B - Give the relationship between the following pairs...Ch. 1 - a. Draw the resonance forms for SO2 (bonded OSO)....Ch. 1 - Name the element that corresponds to each...Ch. 1 - Prob. 1.28SPCh. 1 - For each compound, state whether its bonding is...Ch. 1 - a. Both PCl3 and PCl5 are stable compounds Draw...Ch. 1 - Draw a Lewis structure for each species a. N2H4 b....Ch. 1 - Prob. 1.32SPCh. 1 - Prob. 1.33SPCh. 1 - Draw Lewis structures for a. two compounds of...Ch. 1 - Prob. 1.35SPCh. 1 - Some of the following molecular formulas...Ch. 1 - Prob. 1.37SPCh. 1 - Give the molecular formula of each compound shown...Ch. 1 - 1. From what you remember of electronegativities,...Ch. 1 - For each of the following structures, 1. Draw a...Ch. 1 - Prob. 1.41SPCh. 1 - Prob. 1.42SPCh. 1 - Prob. 1.43SPCh. 1 - Prob. 1.44SPCh. 1 - For each pair of ions, determine which on is more...Ch. 1 - Use resonance structures to identify the areas of...Ch. 1 - Prob. 1.47SPCh. 1 - In 1934, Edward A. Doisy of Washington University...Ch. 1 - If the carbon atom in CH2Cl2 were fat. there would...Ch. 1 - Cyclopropane (C3H6, a three-membered ring) is more...Ch. 1 - Prob. 1.51SPCh. 1 - Prob. 1.52SPCh. 1 - In most amines, the nitrogen atom is sp3...Ch. 1 - Predict the hybridization and geometry of the...Ch. 1 - Draw orbital pictures of the pi bonding in the...Ch. 1 - Prob. 1.56SPCh. 1 - Prob. 1.57SPCh. 1 - Which of the following compounds show cis-trans...Ch. 1 - Give the relationships between the following pairs...Ch. 1 - Dimethyl sulfoxide (DMSO) has been used as an...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nucleophilic Aromatic Substitution: What is the product of the reaction? *see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardThe answer here says that F and K have a singlet and a doublet. The singlet and doublet are referring to the H's 1 carbon away from the carbon attached to the OH. Why don't the H's two carbons away, the ones on the cyclohexane ring, cause more peaks on the signal?arrow_forward
- Draw the Birch Reduction for this aromatic compound and include electron withdrawing groups and electron donating groups. *See attachedarrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see imagearrow_forward
- Elimination-Addition: What molecule was determined to be an intermediate based on a “trapping experiment”? *please solve and see imagearrow_forwardShow the correct sequence to connect the reagent to product. * see imagearrow_forwardPredict the final product. If 2 products are made, list which should be “major” and “minor”. **see attachedarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY