
Concept explainers
(a)
Interpretation:
Lewis structure for the given molecule is to be completed.
Concept introduction:
Lewis structures involve only valence electrons. When drawing a Lewis structure, the first step is to calculate the total number of valence electrons. For a complete Lewis structure of a molecule, the atoms must complete their normal valency by bond formation and lone pairs of electrons. Maximum number of covalent bonds formed by any neutral atom with maximum number of lone pairs is
| Atom | Number of bond | Number of lone pairs |
| C | 4 | 0 |
| H | 1 | 0 |
| O | 2 | 2 |
| N | 1 | 1 |
| F | 1 | 3 |
Answer to Problem 1.46P
The complete Lewis structure for the given molecule is
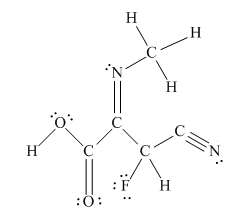
Explanation of Solution
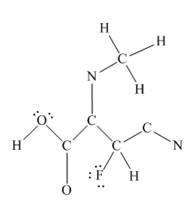
The given structure is
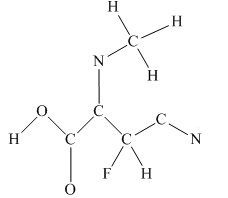
Total valence electron count for the given molecule is
The other oxygen atom has formed only one bond with carbon. This is converted to a double bond and two lone pairs are placed on the oxygen atom so that its octet is complete. A double bond is placed between C and N atom to complete the octet of carbon and a lone pair is placed in nitrogen to complete its octet.
A triple bond is placed between the other C and N to complete the octet of carbon and a lone pair is placed in the nitrogen to complete its octet.

This structure now accounts for all 54 electrons and the octet of each atom, except hydrogen, is complete. The duet for all hydrogens is complete.
The Lewis structure for the given molecule is completed from total valence electron count.
(b)
Interpretation:
Lewis structure for the given molecule is to be completed.
Concept introduction:
Lewis structures involve only valence electrons. When drawing a Lewis structure, the first step is to calculate the total number of valence electrons. For a complete Lewis structure of a molecule, every carbon atom must form four covalent bonds whereas the hydrogen atom forms one bond.
Answer to Problem 1.46P
The complete Lewis structure for the given molecule is
Explanation of Solution
The given structure is
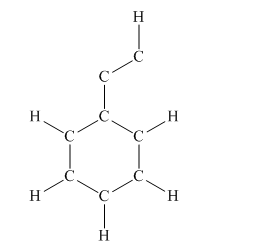
Total valence electron count for the given molecule must be

This structure now accounts for all 38 electrons and the octet of each atom, except hydrogen, is complete. The duet for all hydrogen atoms is complete.
The Lewis structure for the given molecule is completed from total valence electron count.
(c)
Interpretation:
Lewis structure for the given molecule is to be completed.
Concept introduction:
Lewis structures involve only valence electrons. When drawing a Lewis structure, the first step is to calculate the total number of valence electrons. For a complete Lewis structure of a molecule, the atoms must complete their normal valency by bond formation and lone pairs of electrons. Maximum numbers of covalent bonds formed by any neutral atom with maximum number of lone pair are
| Atom | Number of bond | Number of lone pairs |
| C | 4 | 0 |
| H | 1 | 0 |
| O | 2 | 2 |
| N | 1 | 1 |
Answer to Problem 1.46P
The complete Lewis structure for the given molecule is
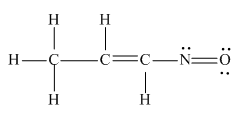
Explanation of Solution
The given structure is
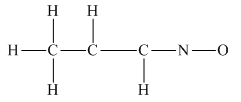
Total valence electron count for the given molecule is
The oxygen atom has formed only one bond with nitrogen. This is converted to a double bond and two lone pairs are placed on the oxygen atom so that its octet is complete. Another lone pair is placed on the nitrogen atom so that its octet is complete.
A double bond is placed between the C atoms attached to one hydrogen each. This completes the octet of both carbon atoms

This structure now accounts for all the 28 electrons, and the octet of each atom, except hydrogen, is complete. The duet for all hydrogen atoms is complete.
The Lewis structure for the given molecule is completed from total valence electron count.
Want to see more full solutions like this?
Chapter 1 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- Explain why this data led Rayleigh to look for and to discover Ar.arrow_forward5) Confidence interval. Berglund and Wichardt investigated the quantitative determination of Cr in high-alloy steels using a potentiometric titration of Cr(VI). Before the titration, samples of the steel were dissolved in acid and the chromium oxidized to Cr(VI) using peroxydisulfate. Shown here are the results (as %w/w Cr) for the analysis of a reference steel. 16.968, 16.922, 16.840, 16.883, 16.887, 16.977, 16.857, 16.728 Calculate the mean, the standard deviation, and the 95% confidence interval about the mean. What does this confidence interval mean?arrow_forwardIn the Nitrous Acid Test for Amines, what is the observable result for primary amines? Group of answer choices nitrogen gas bubbles form a soluble nitrite salt yellow oily layer of nitrosoaminearrow_forward
- 3. a. Use the MS to propose at least two possible molecular formulas. For an unknown compound: 101. 27.0 29.0 41.0 50.0 52.0 55.0 57.0 100 57.5 58.0 58.5 62.0 63.0 64.0 65.0 74.0 40 75.0 76.0 20 20 40 60 80 100 120 140 160 180 200 220 m/z 99.5 68564810898409581251883040 115.0 116.0 77404799 17417M 117.0 12.9 118.0 33.5 119.0 36 133 0 1.2 157.0 2.1 159.0 16 169.0 219 170.0 17 171.0 21.6 172.0 17 181.0 1.3 183.0 197.0 100.0 198.0 200. 784 Relative Intensity 2 2 8 ō (ppm) 6 2arrow_forwardSolve the structure and assign each of the following spectra (IR and C-NMR)arrow_forward1. For an unknown compound with a molecular formula of C8H100: a. What is the DU? (show your work) b. Solve the structure and assign each of the following spectra. 8 6 2 ō (ppm) 4 2 0 200 150 100 50 ō (ppm) LOD D 4000 3000 2000 1500 1000 500 HAVENUMBERI -11arrow_forward
- 16. The proton NMR spectral information shown in this problem is for a compound with formula CioH,N. Expansions are shown for the region from 8.7 to 7.0 ppm. The normal carbon-13 spec- tral results, including DEPT-135 and DEPT-90 results, are tabulated: 7 J Normal Carbon DEPT-135 DEPT-90 19 ppm Positive No peak 122 Positive Positive cus и 124 Positive Positive 126 Positive Positive 128 No peak No peak 4° 129 Positive Positive 130 Positive Positive (144 No peak No peak 148 No peak No peak 150 Positive Positive してしarrow_forward3. Propose a synthesis for the following transformation. Do not draw an arrow-pushing mechanism below, but make sure to draw the product of each proposed step (3 points). + En CN CNarrow_forwardShow work..don't give Ai generated solution...arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

