One method of converting
(b) What about in an isothermal process? (c) What about in an adiabatic process (where heat transfer occurred prior to the adiabatic process)?


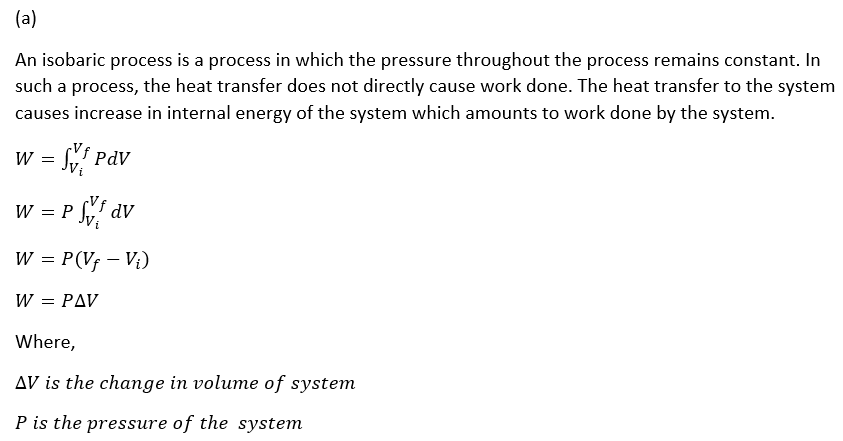
The increase in internal energy leads to an increase in the amount of volume occupied by the gas and hence leading to the movement of piston and the work being done.

Step by step
Solved in 5 steps with 5 images









