My physics class has turned to online due to the COVID-19 and I am having trouble with some of the word problems my teacher gave me to work on. She presented me with: I am contemplating a career change. If I had 500g of stolen gold jewelry, how much energy would I have to add to it to melt it (so that it was not longer recognizable)? Pretend that it is pure gold. Would you be able to show me how to accomplish this problem so I can see the steps? Thank you for your time. John Payton
My physics class has turned to online due to the COVID-19 and I am having trouble with some of the word problems my teacher gave me to work on.
She presented me with: I am contemplating a career change. If I had 500g of stolen gold jewelry, how much energy would I have to add to it to melt it (so that it was not longer recognizable)? Pretend that it is pure gold.
Would you be able to show me how to accomplish this problem so I can see the steps?
Thank you for your time.
John Payton
Given:
Mass of gold (m) = 500 g.
Specific heat of gold (c) = 0.129 J/g ̊C
Latent heat of melting (lf) = 63 J/g
Total heat is given to gold is given as,
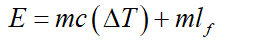
Let the initial temperature of gold (room temperature) (Ti) = 25 ̊C
The melting point of gold (Tf) = 1064 ̊C
Step by step
Solved in 4 steps with 2 images









