Mass flows and specific heat capacity and inlet and outlet temperature for the flows in a heat exchanger are given. Assume that no heat transfer to the surroundings takes
Mass flows and specific heat capacity and inlet and outlet temperature for the flows in a heat exchanger are given. Assume that no heat transfer to the surroundings takes
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
Exercise 1 Entropy change in a heat exchanger
Mass flows and specific heat capacity and inlet and outlet temperature for the flows in a heat exchanger are given. Assume that no heat transfer to the surroundings takes place. Calculate the entropy change in the heat exchanger. Calculate the enthalpy change in the heat exchanger. What is the entropy change in the surroundings? What is the entropy change in the universe? Give the answers in kW/K.
?̇?=5.0kg/s ??,?=1500J/K*kg ??,??=293K ??,out=315K
?̇ℎ=3.0kg/s ??,?=1200J/K*kg ?ℎ,??=343K ?ℎ,out=297K
Expert Solution
The system and the entropy change of the Universe
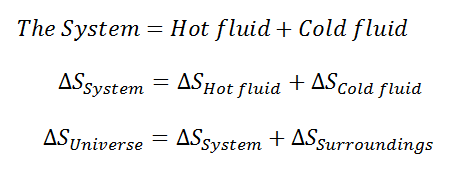

Step by step
Solved in 5 steps with 8 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The