Determine the following for each MS spectrum. 1) Determine the molecular ion peak. 2) Determine if Cl, Br, or S is present in each compound. 3) Use the nitrogen rule to determine if there could be an even or odd number of nitrogen atoms.
Determine the following for each MS spectrum. 1) Determine the molecular ion peak. 2) Determine if Cl, Br, or S is present in each compound. 3) Use the nitrogen rule to determine if there could be an even or odd number of nitrogen atoms.


Different types of spectroscopy can be used to identify the structure of unknown compounds. Some common spectroscopy methods are NMR- spectroscopy, IR-spectroscopy, UV-visible spectroscopy, Mass spectroscopy, etc.
The IR-spectroscopy provides information about the presence of functional groups whereas NMR spectroscopy gives data about types of H present in the molecule.
The molecular ion peak is used to determine the molecular weight of the given compound. It is the peak with the highest value of m/z in the mass spectrum.
In the given spectrum the molecular ion peaks are:
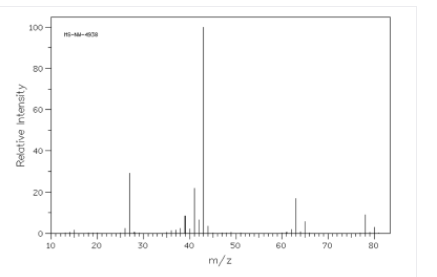 Molecular ion peak = 78
Molecular ion peak = 78
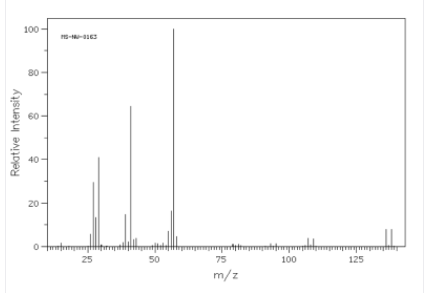 Molecular ion peak = 136
Molecular ion peak = 136
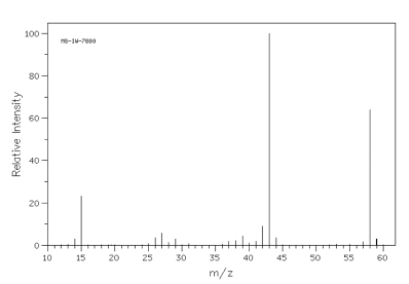 molecular ion peak = 59
molecular ion peak = 59
Step by step
Solved in 6 steps with 8 images









