I have attached two images both are charts for a % salt solution experiment. In the second image the chart is incomplete. I need to know how to find the % salt using the chart from the 1st images. Please show hiw to get answers. Thanks in advance
I have attached two images both are charts for a % salt solution experiment. In the second image the chart is incomplete. I need to know how to find the % salt using the chart from the 1st images. Please show hiw to get answers. Thanks in advance


Percent concentration by mass is the mass of solute divided by the total mass of the solution and multiplied by 100%
The formula to calculate the % salt solution is given in the table 2.
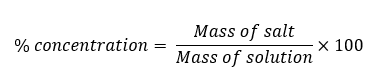
Values for mass of salt and the mass of the solution is what you have to put from table 1. Value for mass of salt is from column 5 (actual mass) and the mass of solution from the last column in the table 1.
The set up in the 1st row in table 2 is the correct.
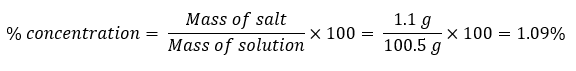
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 5 images









