At a certain temperature, the equilibrium constant K for the following reaction is 5.36 × 10*": H,(g) + Cl,(g) =2 HCI(g) Use this information to complete the following table. Suppose a 33. L reaction vessel is filled with 0.63 mol of HCI. What can you say about the composition of the mixture in the vessel at equilibrium? O There will be very little H2 and Cl2. O There will be very little HCI. O Neither of the above is true. What is the equilibrium constant for the following reaction? Round your answer to 3 significant digits. = ] K = 2 HCl(g) H,(9)+Cl,(9) What is the equilibrium constant for the following reaction? Round your answer to 3 significant digits. K = ] 2 H,(9)+2Cl,(9) 4 HCl(g)
At a certain temperature, the equilibrium constant K for the following reaction is 5.36 × 10*": H,(g) + Cl,(g) =2 HCI(g) Use this information to complete the following table. Suppose a 33. L reaction vessel is filled with 0.63 mol of HCI. What can you say about the composition of the mixture in the vessel at equilibrium? O There will be very little H2 and Cl2. O There will be very little HCI. O Neither of the above is true. What is the equilibrium constant for the following reaction? Round your answer to 3 significant digits. = ] K = 2 HCl(g) H,(9)+Cl,(9) What is the equilibrium constant for the following reaction? Round your answer to 3 significant digits. K = ] 2 H,(9)+2Cl,(9) 4 HCl(g)
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 62QRT
Related questions
Question
![At a certain temperature, the equilibrium constant K for the following reaction is 5.36 x 10":
H,(g) + Cl,(g) = 2 HCI(g)
Use this information to complete the following table.
Suppose a 33. L reaction vessel is filled with 0.63 mol of HCI.
What can you say about the composition of the mixture in the
vessel at equilibrium?
O There will be very little H2 and Cl2.
O There will be very little HCI.
O Neither of the above is true.
What is the equilibrium constant for the following reaction?
Round your answer to 3 significant digits.
K = ]
2 HCl(g)
H,(9)+Cl,(9)
What is the equilibrium constant for the following reaction?
Round your answer to 3 significant digits.
K = ]
2 H,(9)+2Cl,(9)
4 HCl(g)
Continue
O 2022 McGraw Hill LLC. All Rights Reserve
43,203
FEB
18
9 PP
SESS](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fb3b3ba64-2f6b-4003-9bed-e0cda267d11d%2F8b4dc680-fdfd-402e-b1b3-29efdd48b5e0%2Fopt36x_processed.jpeg&w=3840&q=75)
Transcribed Image Text:At a certain temperature, the equilibrium constant K for the following reaction is 5.36 x 10":
H,(g) + Cl,(g) = 2 HCI(g)
Use this information to complete the following table.
Suppose a 33. L reaction vessel is filled with 0.63 mol of HCI.
What can you say about the composition of the mixture in the
vessel at equilibrium?
O There will be very little H2 and Cl2.
O There will be very little HCI.
O Neither of the above is true.
What is the equilibrium constant for the following reaction?
Round your answer to 3 significant digits.
K = ]
2 HCl(g)
H,(9)+Cl,(9)
What is the equilibrium constant for the following reaction?
Round your answer to 3 significant digits.
K = ]
2 H,(9)+2Cl,(9)
4 HCl(g)
Continue
O 2022 McGraw Hill LLC. All Rights Reserve
43,203
FEB
18
9 PP
SESS
Expert Solution
Step 1
The equilibrium reaction given is,
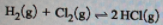
Given: K = 5.36 1011
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning