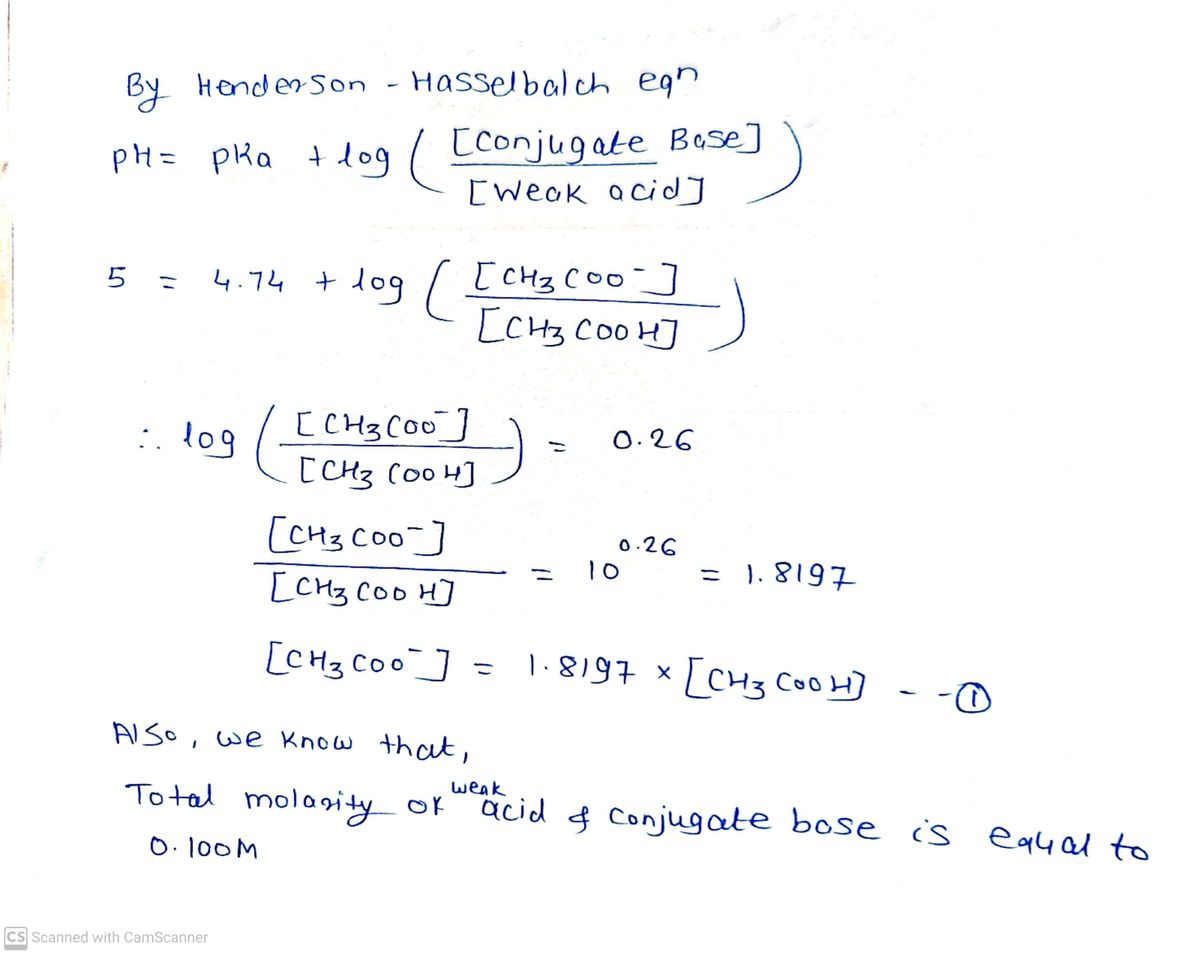
A beaker with 1.40x102 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The total molarity of acid and conjugate base in this buffer is 0.100 M. A student adds 7.00 mL of a 0.490 M HCl solution to the beaker. How much will the pH change? The pKa of acetic acid is 4.740. Express your answer numerically to two decimal places. Use a minus ( - ) sign if the pH has decreased. • View Available Hint(s) ΑΣφ ApH =
A beaker with 1.40x102 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The total molarity of acid and conjugate base in this buffer is 0.100 M. A student adds 7.00 mL of a 0.490 M HCl solution to the beaker. How much will the pH change? The pKa of acetic acid is 4.740. Express your answer numerically to two decimal places. Use a minus ( - ) sign if the pH has decreased. • View Available Hint(s) ΑΣφ ApH =
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question

Transcribed Image Text:A beaker with 1.40x102 mL of an acetic acid buffer with a pH of 5.000 is sitting on a benchtop. The
total molarity of acid and conjugate base in this buffer is 0.100 M. A student adds 7.00 mL of a
0.490 M HCl solution to the beaker. How much will the pH change? The pKa of acetic acid is
4.740.
Express your answer numerically to two decimal places. Use a minus ( - ) sign if the pH
has decreased.
View Available Hint(s)
ΑΣφ
ApH =
Expert Solution
Step 1
Step by step
Solved in 4 steps with 4 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON