7. For each of the following pairs of reactions a. Sketch a comparative Gibbs Free Energy diagram that is illustrative of the different rates observed for each SN2 reaction under the given conditions. Note that if the reaction has more than one mechanistic step (ie; an acid-base reaction or metal insertion) the GFE diagram and rates should only be compared with regard to the one-step Sn2 reaction. Be sure to label your axes and species. b. Concisely rationalize the energy difference(s) identified in part (a). c. Provide the major product for the faster reaction. No mechanism is needed, although you may find it helpful to use electron-pushing arrows to determine the major product. NaSEt THE NaOEt THE
For each of the following pairs of reactions:
a. sketch a comparative gibbs free energy diagram this is illustrative of the different rates observed for each Sn2 reaction under the given conditions. note that if the reaction has more than one mechanism step (i.e. an acid base reaction or metal insertion) the Gibbs Free Energy diagram and rates should only be compared with regard to the one-step Sn2 reaction. Be sure to label your axes and species.
b. concisely rationalize the energy difference(s) identified in part (a).
c. provide the major product for the faster reaction. no mechanism is needed, although you may find it helpful to use electron pushing arrows to determine the major product.

The Gibbs free energy diagram is obtained by plotting the change in free energy of the reaction against the progress of the reaction. The free energy value that corresponds to the top of the peak shows the high energy state of the molecule mostly transition state. In a bimolecular substitution reaction, the rate of the reaction depends on the concentration of the substrate and the nucleophile. The higher the nucleophilicity results in the increase of the rate of the substitution reaction. The major product of a chemical reaction is the one that is obtained in a larger yield due to the higher rate of the step that leads to the product.
a) Let there no observable change in the change in the free energy of the reactants and the products in the reaction of the secondary alkyl chloride with the nucleophiles such as and . The energy profile diagram of both reactions are shown below:
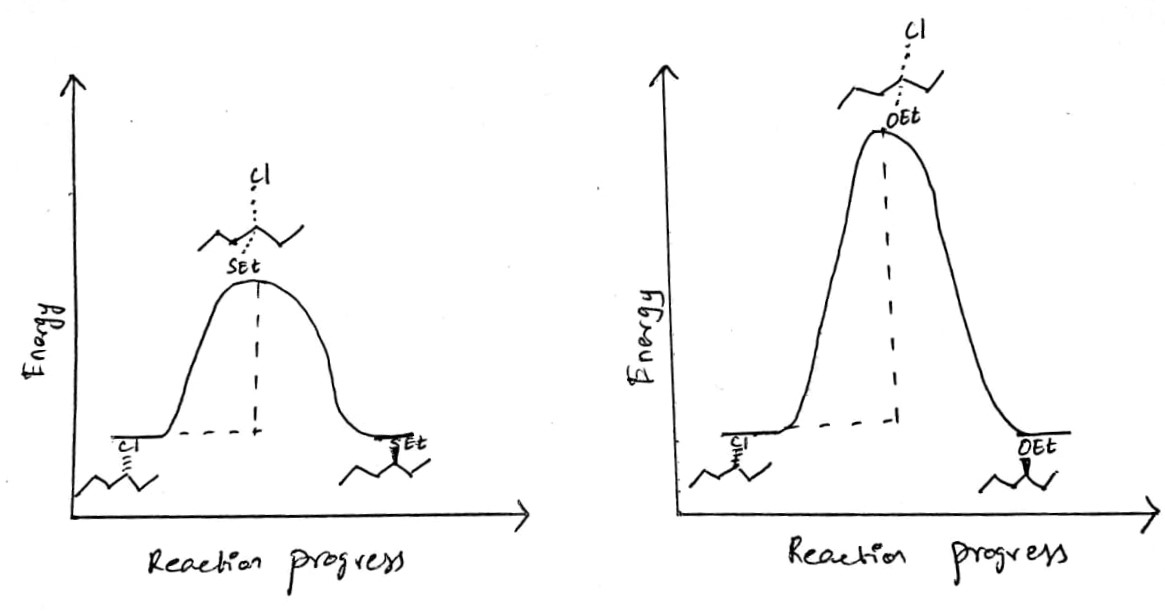
b) The nucleophilicity order of the ions:
The higher nucleophilicity of the thioethoxide ion is due to the negative charge is highly dispersed on the larger sulfur atom than the charge on the smaller and highly electronegative oxygen atom. When the leaving group is identical such as chloride ion, the rate of the reaction is greater for the ion. Hence the Gibbs free energy of the substitution reaction of the thioethoxide ion is lower and its transition can be easily attained than the same reaction with ethoxide ion.
c) Major product:
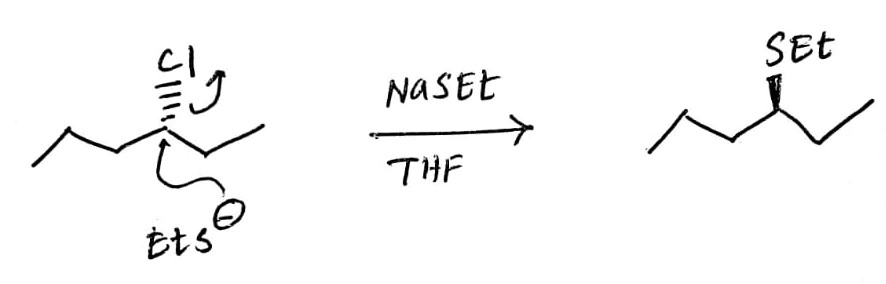
The back side attack of the nucleophile ion removes chloride ion to furnish the alkyl thio ether in which -SEt group is represented in the wedge notation.
Step by step
Solved in 4 steps with 6 images









