18 During a chemical reaction, the concentration of Species A was measured as a function of time. The observed concentration of Species A at various tin intervals is presented in the table below. Determin reaction order and rate constant k and list the appropriate units. Is Species A being removed or
18 During a chemical reaction, the concentration of Species A was measured as a function of time. The observed concentration of Species A at various tin intervals is presented in the table below. Determin reaction order and rate constant k and list the appropriate units. Is Species A being removed or
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question

Transcribed Image Text:**Transcription for Educational Website**
**Chemical Reaction Analysis**
During a chemical reaction, the concentration of Species A was measured as a function of time. The observed concentrations of Species A at various time intervals are presented in the table below. Determine the reaction order and rate constant \( k \) and list the appropriate units. Is Species A being removed or produced?
| Time (min) | Concentration of A (mg/L) |
|------------|---------------------------|
| 0 | 105 |
| 10 | 78 |
| 20 | 62 |
| 30 | 40 |
| 40 | 21 |
| 50 | 1 |
**Table Explanation:**
The table shows the concentration of Species A in milligrams per liter (mg/L) at different time intervals given in minutes (min). The data indicate a decrease in the concentration of Species A over time, suggesting that Species A is being removed during the reaction.
To analyze this data:
1. **Determine the Reaction Order**: Evaluate how the concentration change correlates with time.
2. **Calculate the Rate Constant \( k \)**: Using the reaction order, compute the rate constant and include correct units.
3. **Conclude if Species A is Removed or Produced**: Based on the concentration trend, acknowledge the removal or generation of Species A.
This kind of analysis helps understand the kinetics of the reaction and predict how the concentration of reactants and products change over time.
Expert Solution
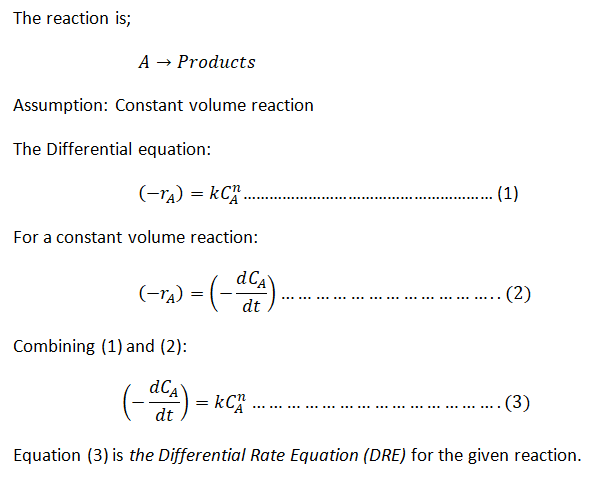
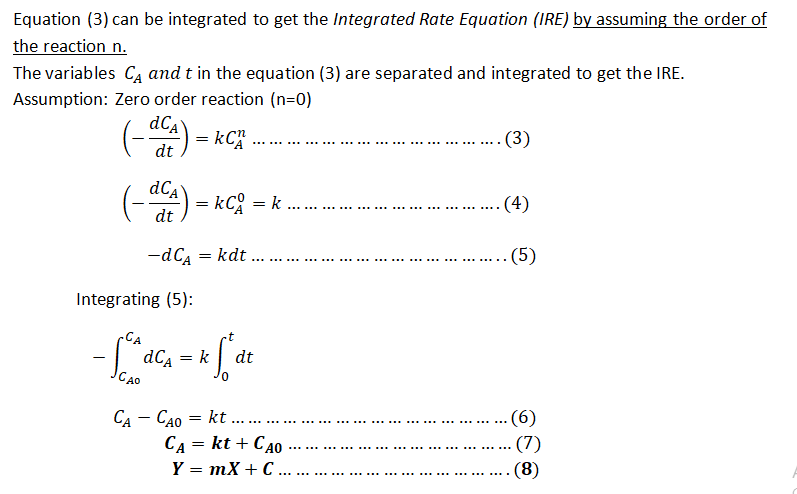
Step 1: Differential Rate Equation(DRE) and the Integrated Rate Equation (IRE)
Step by step
Solved in 4 steps with 5 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The