
Concept explainers
Arrange the compounds of each of the following series in order of increasing acidity:
Interpretation:
The compounds of each of the given series are to be arranged in order of increasing acidity.
Concept introduction:
Acidity depends on the
Resonance structures are the structures in which two or more possible electron structures are drawn. In the resonance structure, the position of the atoms is the same but position of the electrons is different.
Resonance causes delocalization of electron pairs, which increases the stability of the base.
Answer to Problem 1P
Solution:
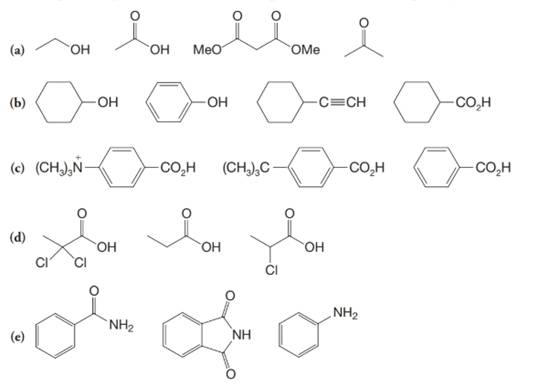
(a)
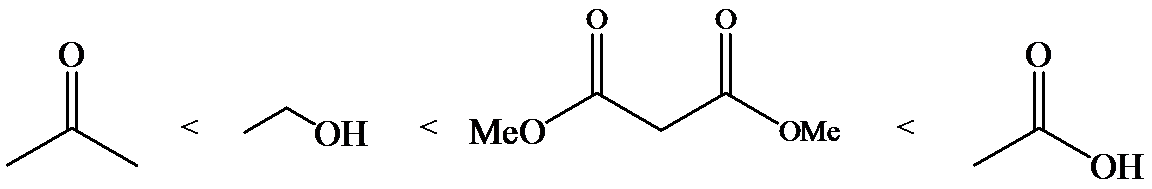
(b)
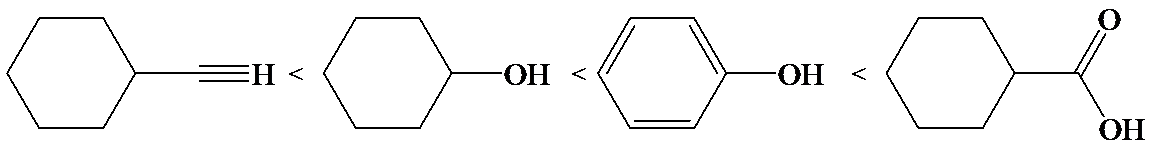
(c)

(d)
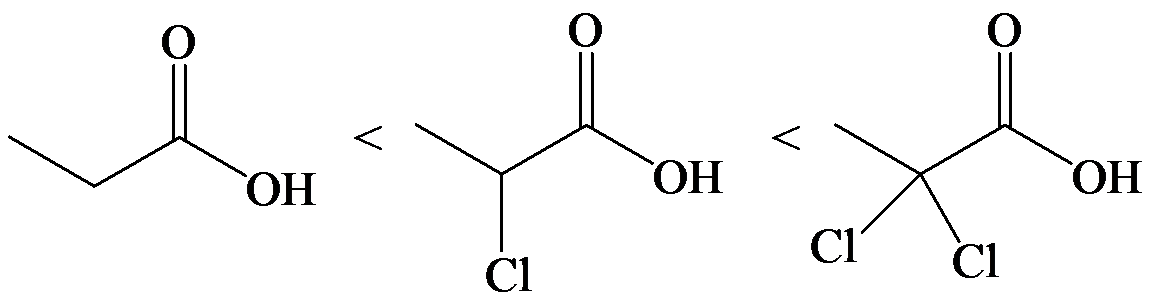
(e)
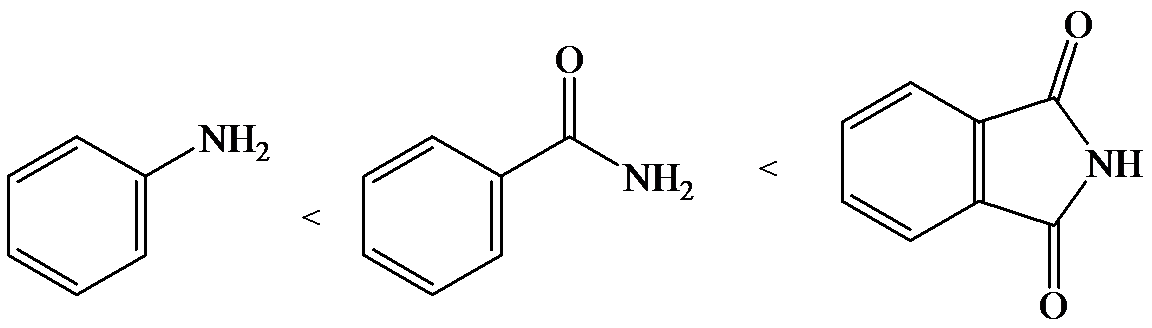
Explanation of Solution
a)
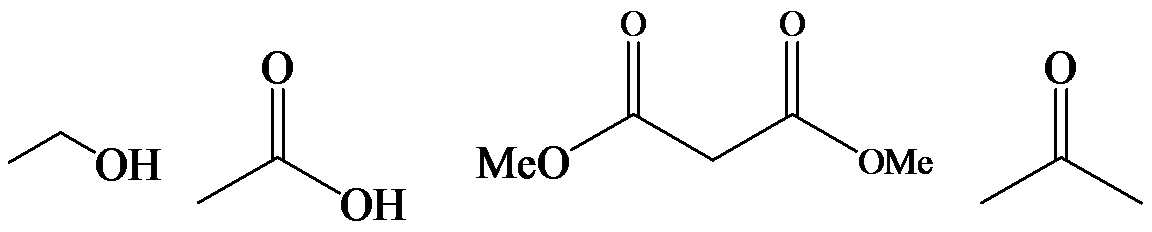
The α-hydrogen atoms of carbonyl groups are acidic. The acidity arises from the electron withdrawing effect of the carbonyl and resonance stabilization of its conjugate base. The electron donating effect of 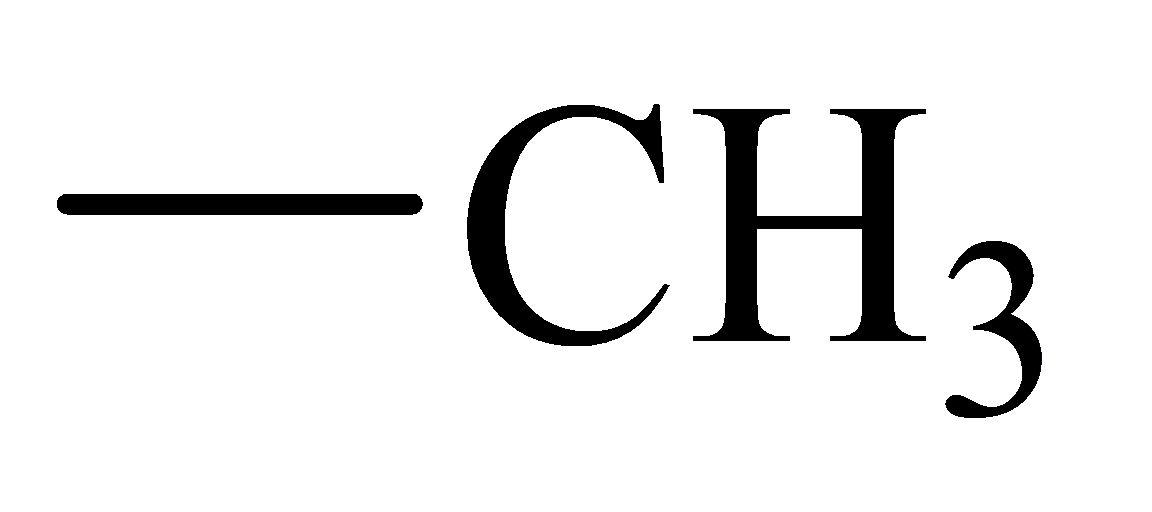 groups tends to destabilize anions. In diketone, there is an active methylene, adjacent to two carbonyl groups. This indicates more resonance stabilization. The charge of anion can be delocalized to both oxygen atoms. The hydroxyl proton in carboxylic acid is an α-proton. On comparing the acidity of carboxylic acids and alcohols, alcohol is less acidic than carboxylic acid.
groups tends to destabilize anions. In diketone, there is an active methylene, adjacent to two carbonyl groups. This indicates more resonance stabilization. The charge of anion can be delocalized to both oxygen atoms. The hydroxyl proton in carboxylic acid is an α-proton. On comparing the acidity of carboxylic acids and alcohols, alcohol is less acidic than carboxylic acid.
So, the increasing order of the acidity is as follows:
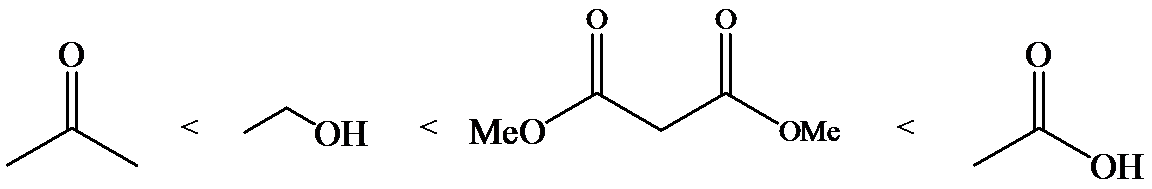
b)
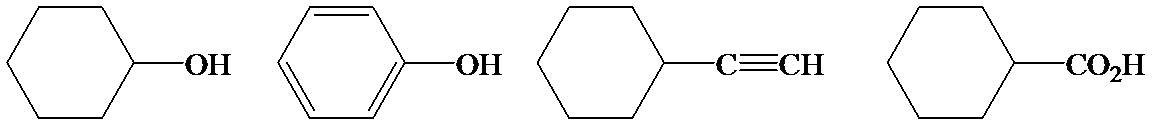
Phenol is more acidic than cyclohexanol because the resonance stabilization in both is different.
In the case of cyclohexane carboxylic acid, negative charge is shared between two different oxygen atoms making it more stabilized than phenoxide. Hence, the removal of proton from cyclohexane carboxylic acid is easier than phenol, making it more acidic than phenol.
So, the increasing order of the acidity is as follows:
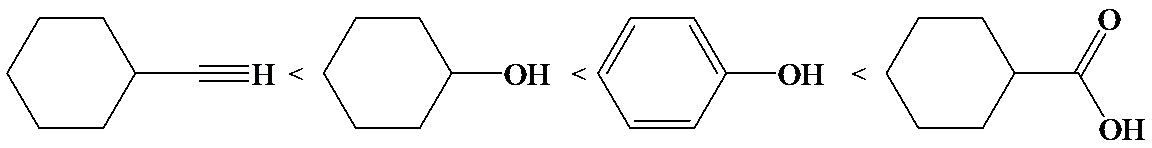
c)
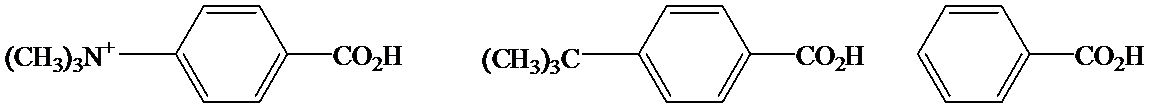
In the case of carboxylic acids, electron substituents increase acidity by inductive electron donation. The electron-donating tert-butyl group destabilizes the conjugate base of benzoic acid, making it less acidic.
So, the increasing order of the acidity is as follows:

d)
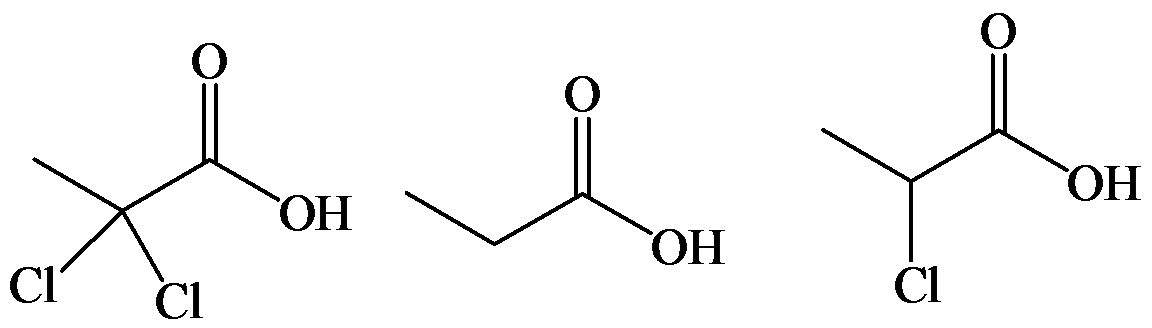
The electron-withdrawing chloro groups increase the acidity of carboxylic acid by increasing the stability of the carboxylate ion. Hence, the carboxylic acid with more chloro groups is more acidic.
So, the increasing order of the acidity is as follows:
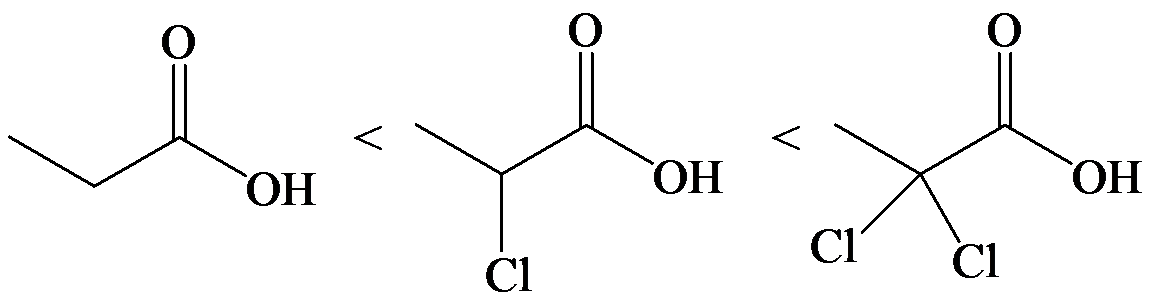
e)
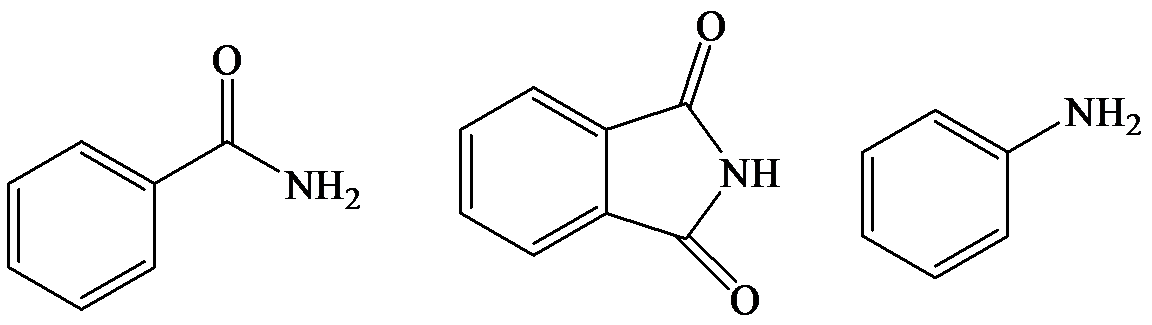
The lone pair electron in aniline is localized on the nitrogen atom, whereas onbenzamide, it is delocalized between oxygen and nitrogen via resonance. Therefore, benzamide is more acidic than aniline.
So, the increasing order of the acidity is as follows:
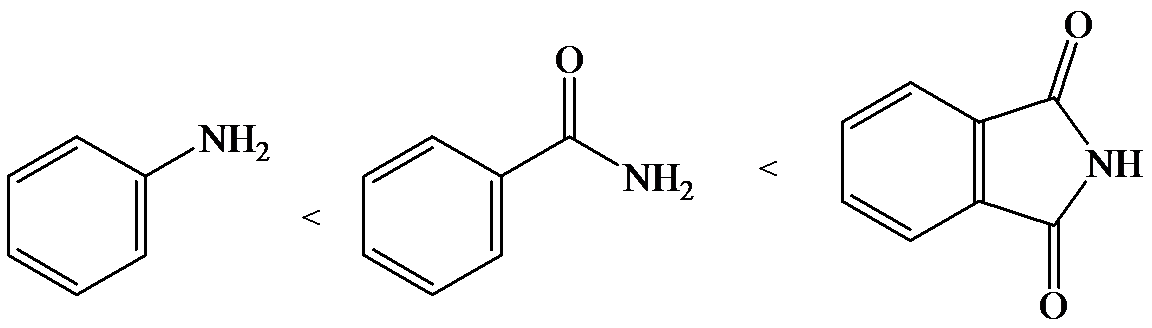
Want to see more full solutions like this?
Chapter SRP Solutions
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
Additional Science Textbook Solutions
Chemistry: Structure and Properties (2nd Edition)
Human Anatomy & Physiology (2nd Edition)
Microbiology: An Introduction
Human Biology: Concepts and Current Issues (8th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Cosmic Perspective Fundamentals
- 18. Arrange the following carbocations in order of decreasing stability. 1 2 A 3124 B 4213 C 2431 D 1234 E 2134 SPL 3 4arrow_forwardAcetic acid is added to DI water at an initial concentration of 10 -6 M (Ka=1.8x10-5) A. Using the "ICE" Method, what would the pH be at equilibrium? State assumptions and show your work. B. Using the simultaneous equations method, what would the pH be at equilibrium? Show your workarrow_forward1. Show that the change in entropy for a fixed amount of ideal gas held at a constant temperature undergoing a volume change is given by the simple equation AS = NkB In Hint: Start with the equation M dS = du + (Œ) dv - Ž (#) an, dU du+av-dN; j=1 Why doesn't the equation for the entropy of an ideal gas depend on the strength of the intermolecular forces for the gas?arrow_forward
- 2. Make an ice cube at 1 bar pressure by freezing an amount of liquid water that is 2 cm x 2 cm x 2 cm in volume. The density of liquid water at 0 °C is 1.000 g cm³ and the density of ice at 0 °C is 0.915 g cm³. Note that this difference in density is the reason your water pipes burst if they freeze and why you shouldn't forget to take your bottle of pop out of the freezer if you put it in there to try and cool it down faster. A. What is the work of expansion upon freezing? B. Is work done on the system or by the system?arrow_forwardI have a excitation/emission spectra of a quinine standard solution here, and I'm having trouble interpreting it. the red line is emission the blue line is excitation. i'm having trouble interpreting properly. just want to know if there is any evidence of raman or rayleigh peaks in the spectra.arrow_forwardGive the major product of the following reaction. excess 1. OH, H₂O 1.OH H CH3CH2CH21 H 2. A.-H₂O Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Template toolbars. The single bond is active by default.arrow_forward
- 2. Use Hess's law to calculate the AH (in kJ) for: rxn CIF(g) + F2(g) → CIF 3 (1) using the following information: 2CIF(g) + O2(g) → Cl₂O(g) + OF 2(g) AH = 167.5 kJ ΔΗ 2F2 (g) + O2(g) → 2 OF 2(g) 2C1F3 (1) + 202(g) → Cl₂O(g) + 3 OF 2(g) о = = -43.5 kJ AH = 394.1kJarrow_forwardci Draw the major product(s) of the following reactions: (3 pts) CH3 HNO3/H2SO4 HNO3/ H2SO4 OCH3 (1 pts)arrow_forwardProvide the product for the reactionarrow_forward
- What is the net ionic equation for the reaction between tin(IV) sulfide and nitric acid?arrow_forwardThe combustion of 28.8 g of NH3 consumes exactly _____ g of O2. 4 NH3 + 7 O2 ----> 4 NO2 + 6 H2Oarrow_forwardWhat is the molecular formula of the bond-line structure shown below OH HO ○ C14H12O2 ○ C16H14O2 ○ C16H12O2 O C14H14O2arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning




