
EBK ORGANIC CHEMISTRY
12th Edition
ISBN: 9781119233664
Author: Snyder
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter SRP, Problem 10P
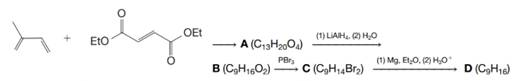
Give stereochemical structures for compounds A–D:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Why doesn't this carry on to form a ring by deprotonating the alpha carbon and the negatively-charged carbon attacking the C=O?
6. A solution (0.0004 M) of Fe(S2CNEt2)3 (see the structural drawing below) in chloroform
has absorption bands at:
350 nm (absorbance A = 2.34);
514 nm(absorbance A = 0.0532);
Calculate the molar absorptivity values for these bands. Comment
on their possible nature (charge transfer transitions or d-d
S
N-
transitions?).
(4 points)
What is the mechanism for this?
Chapter SRP Solutions
EBK ORGANIC CHEMISTRY
Ch. SRP - 1. Arrange the compounds of each of the following...Ch. SRP - 2. Arrange the compounds of each of the following...Ch. SRP - Predict the final product from each of the...Ch. SRP - Prob. 4PCh. SRP - Write detailed mechanisms for each of the...Ch. SRP - Prob. 6PCh. SRP - Prob. 7PCh. SRP - Prob. 8PCh. SRP - Prob. 9PCh. SRP - Give stereochemical structures for compounds AD:
Ch. SRP - Prob. 11PCh. SRP - The remaining steps in the industrial synthesis of...Ch. SRP - Prob. 13PCh. SRP - Prob. 14PCh. SRP - Prob. 15PCh. SRP - Prob. 16PCh. SRP - 17. Show how you would modify the synthesis given...Ch. SRP - Prob. 18PCh. SRP - Give structures for compounds AD. Compound D gives...Ch. SRP - The tranquilizing drug meprobamate (Equanil or...Ch. SRP - Prob. 21PCh. SRP - 22. Outlined here is the synthesis of a central...Ch. SRP - 23. What are compounds A and B? Compound B has a...Ch. SRP - Prob. 24PCh. SRP - 25. The Dow process for synthesizing phenol, which...Ch. SRP - Prob. 26PCh. SRP - Prob. 27PCh. SRP - 28. Compound Y shows prominent IR absorption...Ch. SRP - Prob. 29PCh. SRP - Consider this reaction involving peracetic acid:...Ch. SRP - 31. A compound (N) with the molecular formula...Ch. SRP - 32. Compound X is insoluble in aqueous sodium...Ch. SRP - Write the structures of the three products...Ch. SRP - Compound C (C9H11NO) gives a positive Tollens test...Ch. SRP - A compound X (C10H14O) dissolves in aqueous sodium...Ch. SRP - Compound Z (C5H10O) decolorizes bromine. The IR...Ch. SRP - 37. Compound W was isolated from a marine annelid...Ch. SRP - 38. Phenols generally are not changed on treatment...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Which of the roll owing compounds have a dipole moment of zero?
Organic Chemistry (8th Edition)
Flask A contains yeast cells in glucose-minimal salts broth incubated at 30C with aeration. Flask B contains ye...
Microbiology: An Introduction
6.1 State the number of electrons that be must be lost by atoms of each of the following to achieve a stable el...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
l. FIGURE Q26.1 shows the x-component of as a function of x. Draw a graph of V versus x in this same region of ...
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
8. A human maintaining a vegan diet (containing no animal products) would be a:
a. producer
b. primary consume...
Human Biology: Concepts and Current Issues (8th Edition)
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For questions 1-4, consider the following complexes: [Co(CN)6], [COC14]², [Cr(H2O)6]²+ 4. Room temperature (20°C) measurement of molar magnetic susceptibility (Xm) for Fe(NH4)2(SO4)2×6H2O is 1.1888 x 102 cgs (Gaussian units). Calculate effective magnetic moment and provide a number of unpaired electrons for the iron ion. Use this number to rationalize the coordination geometry around iron center. (4 points)arrow_forward7. Describe the expected 31P and 19F (where applicable) NMR spectral patterns for the following compounds (indicate number of signals and their splitting patterns). a) tetraphenyldiphosphine Ph Ph P-P Ph Ph Ph Ph ' b) tetraphenyldiphosphine monoxide P-P-Ph Ph (2 points) (2 points c) tetrafluorophosphonium hexafluorophosphate [PF4]*[PF6]¯ (4 points)arrow_forward3. For questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ Which (if any) of these complexes would be expected to display Jahn-Teller distortion? (2 points)arrow_forward
- What is Instrumental Neutron Activation and what are the advantages and disadvantages in using its applications? (I'm doing an in class assignment and need better understanding of what the instrument can be used for) Please include references so that I can better understand the application of how the instrument works!arrow_forwardWhat is Isotope Analysis and what are the advantages and disadvantages in using its applications and instrumentalization? Please include references so that I can better understand how the instrument works!arrow_forward5. Count the electrons on the following complexes and state whether they follow the 18- electron rule: (3 points) Fe(CO)5 Ni(PMe3)4 PMe3 is trimethylphosphine Mn(CO)5Brarrow_forward
- For questions 1-4, consider the following complexes: [Co(CN)6]+, [CoCl4]², [Cr(H2O)6]²+ 2. Draw the corresponding d-orbital splitting for each of the complexes; predict the spin- state (low-spin/high spin) for each of the complexes (if applicable); explain your arguments. Calculate the crystal field stabilization energy for each complex (in Ao or At). (6 points)arrow_forwardFor questions 1-4, consider the following complexes: [Co(CN)6]4, [COC14]², [Cr(H2O)6]²+ 1. Assign oxidation number to the metal, then indicate d-electron count. (3 points)arrow_forwardUsing iodometry I want to titrate a sodium thiosulfate solution and I use 15 mL. If I have 50 mL of a 0.90 M copper solution and KI, what will be the molarity of sodium thiosulfate?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY