
Concept explainers
(a)
Interpretation:
The correct structure is to be drawn from the given IUPAC name of the molecule.
Concept introduction:
The structure of a compound can be drawn on the basis of its IUPAC name. The IUPAC name is made of three parts, prefix, root, and suffix. The suffix indicates the highest priority group present. Its location is written as a prefix for the functional group name unless redundant. The root is the longest continuous carbon chain that also includes the highest priority functional group. Any other
If any chiral carbons are present, their absolute configurations are specified at the start along with the carbon number if necessary. Similarly, the stereochemistry (E/Z) of any double bond is also specified at the start. A prefix di, tri, etc., before a prefix or suffix indicates the number of instances of that functional group.
Answer to Problem F.6P
The correct structure of
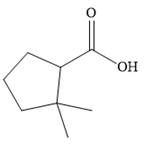
Explanation of Solution
The compound is a
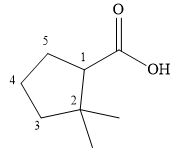
The methyl groups are then attached to the next ring carbon.
Therefore, the structure of

The structure of the compound can be drawn from its IUPAC name, which lists the functional groups attached to an alkyl/aryl root (parent).
(b)
Interpretation:
The correct structure is to be drawn from the given IUPAC name of molecule.
Concept introduction:
The structure of a compound can be drawn on the basis of its IUPAC name. The IUPAC name is made of three parts, prefix, root, and suffix. The suffix indicates the highest priority group present. Its location is written as a prefix for the functional group name unless redundant. The root is the longest continuous carbon chain that also includes the highest priority functional group. Any other functional groups present are listed alphabetically as prefixes along with their locants.
If any chiral carbons are present, their absolute configurations are specified at the start along with the carbon number if necessary. Similarly, the stereochemistry (E/Z) of any double bond is also specified at the start. A prefix di, tri, etc., before a prefix or suffix indicates the number of instances of that functional group.
Answer to Problem F.6P
The structure of
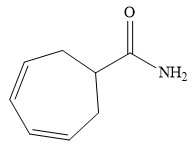
Explanation of Solution
The name shows that the root is a seven carbon ring with two double bonds. The root name is, therefore, cycloheptadiene. The only functional group is an amide (
Thus, the structure of

The structure of the compound can be drawn from its IUPAC name, which lists the functional groups attached to an alkyl/aryl root (parent).
(c)
Interpretation:
The correct structure of
Concept introduction:
The structure of a compound can be drawn on the basis of its IUPAC name. The IUPAC name is made of three parts, prefix, root, and suffix. The suffix indicates the highest priority group present. Its location is written as a prefix for the functional group name unless redundant. The root is the longest continuous carbon chain that also includes the highest priority functional group. Any other functional groups present are listed alphabetically as prefixes along with their locants.
If any chiral carbons are present, their absolute configurations are specified at the start along with the carbon number if necessary. Similarly, the stereochemistry (E/Z) of any double bond is also specified at the start. A prefix di, tri, etc., before a prefix or suffix indicates the number of instances of that functional group.
Answer to Problem F.6P
The structure of
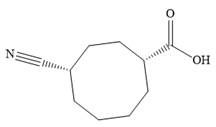
Explanation of Solution
The name shows that the root is an eight carbon ring with two functional groups directly attached to ring carbons. The highest priority group is a carboxylic acid group, and the corresponding ring carbon is numbered 1. The second, nitrile group, is then on C4.
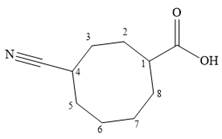
Both C1 and C4 are chiral carbons with R and S configurations respectively. According to Cahn-Ingold-Prelog rules, this means the priority groups 1 to 3 attached to C1 are arranged clockwise if the lowest priority group H is pointing away from the observer, or counterclockwise if H is pointing toward the observer. The priority groups 1 to 3 attached to C4 are arranged conterclockwise if the lowest priority H is pointing away, or clockwise if it is pointing toward the observer.
The structure of the compound can, therefore, be drawn as:
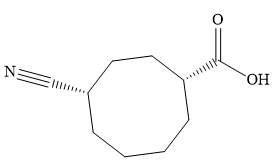
The structure of the compound can be drawn from its IUPAC name, which lists the functional groups attached to an alkyl/aryl root (parent).
(d)
Interpretation:
The structure of
Concept introduction:
The structure of a compound can be drawn on the basis of its IUPAC name. The IUPAC name is made of three parts, prefix, root, and suffix. The suffix indicates the highest priority group present. Its location is written as a prefix for the functional group name unless redundant. The root is the longest continuous carbon chain that also includes the highest priority functional group. Any other functional groups present are listed alphabetically as prefixes along with their locants.
If any chiral carbons are present, their absolute configurations are specified at the start along with the carbon number if necessary. Similarly, the stereochemistry (E/Z) of any double bond is also specified at the start. A prefix di, tri, etc., before a prefix or suffix indicates the number of instances of that functional group.
Answer to Problem F.6P
The correct structure of
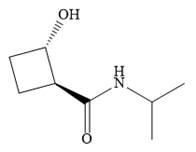
Explanation of Solution
The name shows that the root is a cyclobutane ring. Two functional groups are attached to the ring, a secondary amide and a hydroxyl group. The amide is the higher priority group, so the ring atoms are numbered from the carbon to which it is attached. The hydroxyl group is then attached to the adjacent carbon C2.
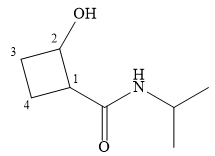
C1 and C2 are both chiral carbons with an absolute configuration of S. This means on each of these carbons, the priority 1 to 3 groups are arranged counterclockwise with the lowest priority H pointing away from the observer, or clockwise with the H pointing toward the observer.
The complete structure, using dash-wedge representation, for the two functional groups, can then be drawn as

The structure of the compound can be drawn from its IUPAC name, which lists the functional groups attached to an alkyl/aryl root (parent).
(e)
Interpretation:
The structure of
Concept introduction:
The structure of a compound can be drawn on the basis of its IUPAC name. The IUPAC name is made of three parts, prefix, root, and suffix. The suffix indicates the highest priority group present. Its location is written as a prefix for the functional group name unless redundant. The root is the longest continuous carbon chain that also includes the highest priority functional group. Any other functional groups present are listed alphabetically as prefixes along with their locants.
If any chiral carbons are present, their absolute configurations are specified at the start along with the carbon number if necessary. Similarly, the stereochemistry (E/Z) of any double bond is also specified at the start. A prefix di, tri, etc., before a prefix or suffix indicates the number of instances of that functional group.
Answer to Problem F.6P
The structure of
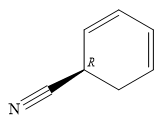
Explanation of Solution
The name shows that the root is a six carbon ring, with two double bonds. The root name is, therefore, cyclohexadiene. One functional group, nitrile, is attached to the ring. The ring
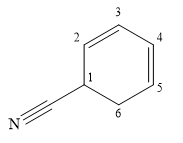
C1 is a chiral carbon with an absolute configuration of R. Therefore, the priority groups 1 to 3 attached to it must be arranged clockwise with the lowest priority hydrogen pointing away from the observer.
Thus, the structure of the compound can be drawn as
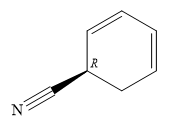
The structure of the compound can be drawn from its IUPAC name, which lists the functional groups attached to an alkyl/aryl root (parent).
Want to see more full solutions like this?
Chapter F Solutions
EBK ORGANIC CHEMISTRY: PRINCIPLES AND M
- Calculate E° for Ni(glycine)2 + 2e– D Ni + 2 glycine– given Ni2+ + 2 glycine– D Ni(glycine)2 K = 1.2×1011 Ni2+ + 2 e– D Ni E° = -0.236 Varrow_forwardOne method for the analysis of Fe3+, which is used with a variety of sample matrices, is to form the highly colored Fe3+–thioglycolic acid complex. The complex absorbs strongly at 535 nm. Standardizing the method is accomplished using external standards. A 10.00-ppm Fe3+ working standard is prepared by transferring a 10-mL aliquot of a 100.0 ppm stock solution of Fe3+ to a 100-mL volumetric flask and diluting to volume. Calibration standards of 1.00, 2.00, 3.00, 4.00, and 5.00 ppm are prepared by transferring appropriate amounts of the 10.0 ppm working solution into separate 50-mL volumetric flasks, each of which contains 5 mL of thioglycolic acid, 2 mL of 20% w/v ammonium citrate, and 5 mL of 0.22 M NH3. After diluting to volume and mixing, the absorbances of the external standards are measured against an appropriate blank. Samples are prepared for analysis by taking a portion known to contain approximately 0.1 g of Fe3+, dissolving it in a minimum amount of HNO3, and diluting to…arrow_forwardAbsorbance and transmittance are related by: A = -log(T) A solution has a transmittance of 35% in a 1-cm-pathlength cell at a certain wavelength. Calculate the transmittance if you dilute 25.0 mL of the solution to 50.0 mL? (A = εbc) What is the transmittance of the original solution if the pathlength is increased to 10 cm?arrow_forward
- Under what conditions will Beer’s Law most likely NO LONGER be linear? When the absorbing species is very dilute. When the absorbing species participates in a concentration-dependent equilibrium. When the solution being studied contains a mixture of ions.arrow_forwardCompared to incident (exciting) radiation, fluorescence emission will have a: Higher energy Higher frequency Longer wavelengtharrow_forwardLin and Brown described a quantitative method for methanol based on its effect on the visible spectrum of methylene blue. In the absence of methanol, methylene blue has two prominent absorption bands at 610 nm and 663 nm, which correspond to the monomer and the dimer, respectively. In the presence of methanol, the intensity of the dimer’s absorption band decreases, while that for the monomer increases. For concentrations of methanol between 0 and 30% v/v, the ratio of the two absorbance, A663/ A610, is a linear function of the amount of methanol. Use the following standardization data to determine the %v/v methanol in a sample if A610 is 0.75 and A663 is 1.07.arrow_forward
- The crystal field splitting energy, Δ, of a complex is determined to be 2.9 × 10-19 What wavelength of light would this complex absorb? What color of light is this? What color would the compound be in solution?arrow_forwardA key component of a monochromator is the exit slit. As the exit slit is narrowed, the bandwidth of light (i.e., the range of wavelengths) exiting the slit gets smaller, leading to higher resolution. What is a possible disadvantage of narrowing the exit slit? (Hint: why might a narrower slit lower the sensitivity of the measurement?).arrow_forwardAn x-ray has a frequency of 3.33 × 1018 What is the wavelength of this light?arrow_forward
- Choose the Lewis structure for the compound below: H2CCHOCH2CH(CH3)2 HH H :d H H H C. Η H H HH H H H H. H H H HH H H H H H- H H H C-H H H HHHHarrow_forwardEach of the highlighted carbon atoms is connected to hydrogen atoms.arrow_forwardく Complete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. More... No reaction. Explanation Check O + G 1. Na O Me Click and drag to start drawing a structure. 2. H + 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 000 Ar Parrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





