
Practice calculating dilutions using the following problems.
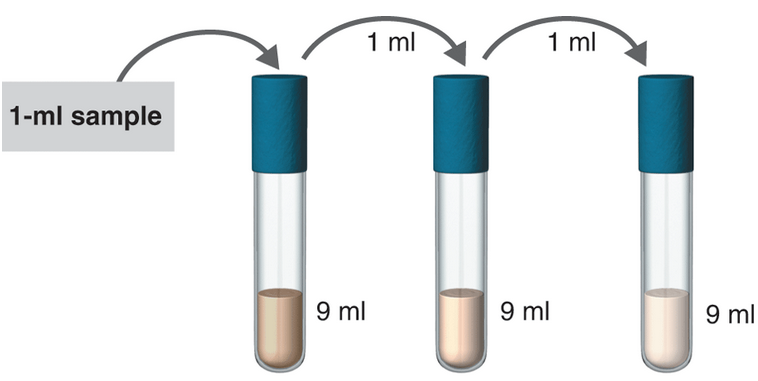
- Dilution:
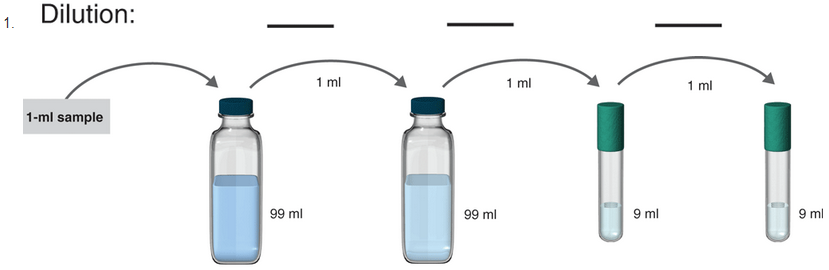
To determine:
The serial dilution in the given samples.
Introduction:
The stepwise dilution of a solution is termed as a serial dilution. Generally, the dilution factor at each stage of serial dilution is constant and leads to geometric progression in a logarithmic manner of the concentration of the sample. Serial dilutions are used to dilute the given substance at a very high level and serial dilutions are also used in the formation of concentration curves.
Explanation of Solution
The samples where serial dilution is occurring is shown below:
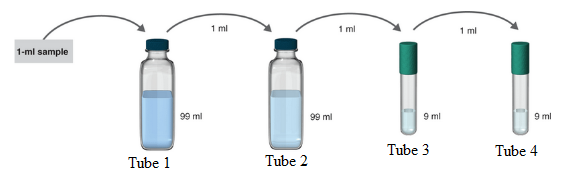
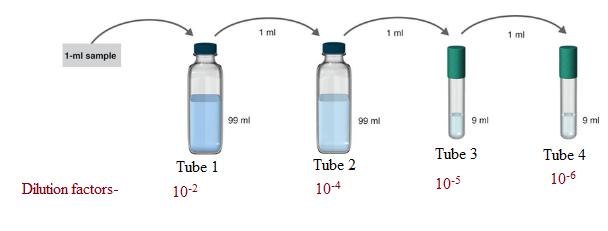
1 ml of the sample is mixed in 99ml solvent. So, the total solution is of 100 ml.
Hence, in tube 1 the serial dilution is of 1:100.
Now, 1 ml of the sample from tube 1 is poured to 99 ml solvent in tube 2. So, the total volume of the solution in tube 2 becomes 100 ml.
Hence, in tube 2 the serial dilution is now =
Now, 1 ml of the sample from tube 2 is poured to 9 ml solvent in tube 3. So, the total volume of the solution in tube 3 becomes 10 ml.
Hence, in tube 3 the serial dilution is now =
Now, 1 ml of the sample from tube 3 is poured to 9 ml solvent in tube 4. So, the total volume of the solution in tube 4 becomes 10 ml.
Hence, in tube 4 the serial dilution is now =
The samples where serial dilution is occurring is shown below:
Want to see more full solutions like this?
Chapter B Solutions
Laboratory Experiments in Microbiology (12th Edition) (What's New in Microbiology)
- If you wanted to reduce the difference between peak and trough levels that occur with repeated administration of a drug, how would you adjust the dose and dose interval without changing the plateau concentration (plateau is the average of peak and trough levels)? Select your answers for both dose and interval. Hint: It may be helpful to think about this problem using an example such as food. How would you eat if you wanted to maintain very steady hunger/satiety levels without changing your total caloric intake? Options: A. Dose; Increase dose B. Dose; Decrease dose C. Dose; Do not change dose D. Interval; Increase the interval between doses (give the drug less frequently) E. Interval; Decrease the interval between doses (give the drug more frequently) F. Interval; Do not change the intervalarrow_forwardWhat percentage of a drug is eliminated after 4 half-lives? Please round to the nearest percent. Show the matharrow_forwardBriefly explain the 6 domain of interprofessional collaboration: Role clarification, Team functioning, Interprofessional communication, Patient/client/family/community-centered care, Interprofessional conflict resolution, Collaborative leadership. Provide a specific negative events that nursing student would observe in a clinical setting for each domain.arrow_forward
- what is an intermittent water course and what kind of fish habitat it would providearrow_forwardwhy are native freshwater mussels are an important part of great lakes ecosystemarrow_forwardwhat morphological features differentiate the lamprey species and other species in the great lakesarrow_forward
- There are a wide range of therapeutic applications available as options for patients. Medical professionals should be aware of these applications so they can make informed recommendations to patients. To gain a better understanding of some therapeutic applications and how they are related to RNA and mRNA, research long non-coding RNA. Respond to the following in a minimum of 175 words: What is lncRNA and what does it do? How does IncRNA differ from mRNA? What are some therapeutic applications associated with lncRNA? Think about possible future uses of this application. What are the advantages and disadvantages of this application and its continued use?arrow_forwardfour fish or mussel species that are native to the great lakesarrow_forwardThere are a wide range of therapeutic applications available as options for patients. Medical professionals should be aware of these applications so they can make informed recommendations to patients. To gain a better understanding of some therapeutic applications and how they are related to RNA and mRNA, research long non-coding RNA. Respond to the following in a minimum of 175 words: What is lncRNA and what does it do? How does IncRNA differ from mRNA? What are some therapeutic applications associated with lncRNA? Think about possible future uses of this application. What are the advantages and disadvantages of this application and its continued use?arrow_forward
- four physial characteristics of a fish or a mussel that would help you identify it to a speciesarrow_forwarddescribe what you would do in this situation, you are working ona. river and it will take 20 minutes by boat to get back to the field truck, you are 1 hour from finishing the field work on the last day of field trip. you hear thunder int he dsitnace, what did you do?arrow_forwardunu grow because auxin is still produced in the tip to Another of Boysen and Jensen's experiments included the use of mica, explain why one of the shoots was able to show phototropism and the other was not. Mica Wafer Ligh c. They then t but this time permeable n shoot. Why phototropis Light Mica Wafer Coleoptile tips Tips removed: agar Explain why the shoo direction after the ag the cut shoot, even tarrow_forward
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning





