
Concept explainers
(a)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
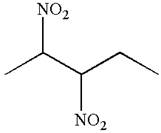
In this molecule, the longest continuous chain of carbon atoms has five carbon atoms. Hence, the root is pentane. The parent chain has two identical substituents; thus the chain is numbered so as to provide the lowest set of locants.
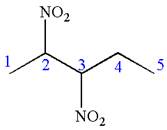
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(b)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms. The name of the substituent attached to the parent chain is written as a prefix to the left side of the root. The chain is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
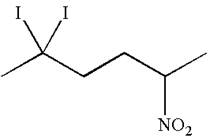
In this molecule, the longest continuous chain of carbon atoms has six carbon atoms. Hence, the root is hexane. The parent chain has three substituents; thus the chain is numbered so as to provide the lowest set of locants.
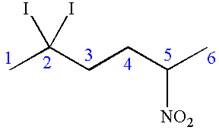
The substituents are arranged in an alphabetical order with respective locant.
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(c)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms. The name of the substituent attached to the parent chain is written as a prefix to the left side of the root. The chain is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
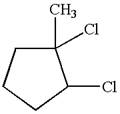
In this molecule, the five carbons ring is a parent. Hence, the root is cyclopentane. The ring has three substituents; thus, the ring is numbered so as to provide the lowest set of locants.
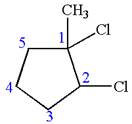
The substituents are arranged in alphabetical order with respective locant.
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(d)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms or the ring structure. The name of the substituent attached to the parent ring is written as a prefix to the left side of the root. The parent ring is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
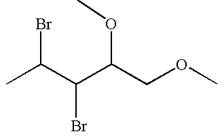
In this molecule, the five carbons ring is a parent. Hence, the root is cyclopentane. The parent chain has four substituents; thus, the chain is numbered so as to provide the lowest set of locants according to alphabetical order.
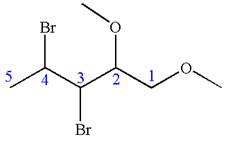
The substituents are arranged in alphabetical order with respective locant.
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(e)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms. The name of the substituent attached to the parent chain is written as a prefix to the left side of the root. The chain is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
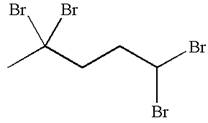
In this molecule, the five carbons ring is a parent. Hence, the root is cyclopentane. The parent chain has four identical substituents; thus, the chain is numbered so as to provide the lowest set of locants according to alphabetical order.
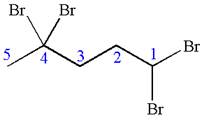
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(f)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms or the ring structure. The name of the substituent attached to the parent ring is written as a prefix to the left side of the root. The parent ring is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents in a way to provide the lowest set of locants.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
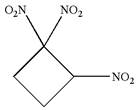
In this molecule, the four carbons ring is a parent. Hence, the root is cyclobutane. The ring has three identical substituents; thus, the ring is numbered so as to provide the lowest set of locants.
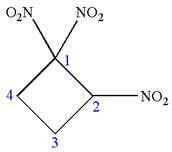
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(g)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms or the ring structure. The name of the substituent attached to the parent ring is written as a prefix to the left side of the root. The parent ring is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
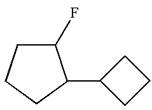
In this molecule, the five carbons ring is a parent. Hence, the root is cyclopentane. The ring has two substituents; thus, the ring is numbered so as to provide the lowest set of locants according to alphabetical order.
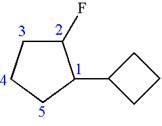
The substituents are arranged in alphabetical order with respective locant.
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(h)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms. The name of the substituent attached to the parent chain is written as a prefix to the left side of the root. The chain is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
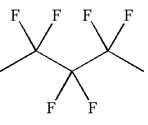
In this molecule, the longest continuous chain of carbon atoms has five carbon atoms. Hence, the root is pentane. The parent chain has six identical substituents; thus, the chain is numbered so as to provide the lowest set of locants.
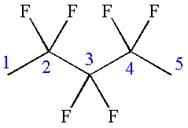
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(i)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms. The name of the substituent attached to the parent chain is written as a prefix to the left side of the root. The chain is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
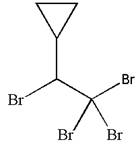
In this molecule, the longest continuous chain of carbon atoms has two carbon atoms. Hence, the root is ethane. The parent chain has five substituents; thus, the chain is numbered so as to provide the lowest set of locants.
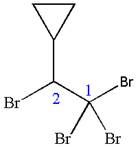
The substituents are arranged in alphabetical order with respective locant.
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
(j)
Interpretation:
The IUPAC name of the given molecule is to be written.
Concept introduction:
The root name of the molecule is the name of the alkane, which depends on the longest continuous chain of carbon atoms. The name of the substituent attached to the parent chain is written as a prefix to the left side of the root. The chain is numbered such that the carbon atom to which the substituent is attached gets the lowest possible number. This number is written on the left side of the substituent and separated by a hyphen. If more than one substituent is present, then the numbering is determined by the alphabetical order of substituents.
Answer to Problem A.46P
The IUPAC name of the given molecule is
Explanation of Solution
The given molecule is
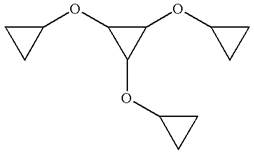
In this molecule, the middle ring of three carbons is a parent. Hence, the root is cyclopropane. The ring has three identical substituents.
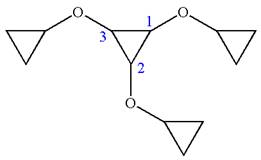
Hence, the IUPAC name is
The IUPAC name of the given molecule is written as
Want to see more full solutions like this?
Chapter A Solutions
EBK GET READY FOR ORGANIC CHEMISTRY
- can someone draw out the reaction mechanism for this reaction showing all the curly arrows and 2. Draw the GPNA molecule and identify the phenylalanine portion. 3. Draw L-phenylalanine with the correct stereochemistryarrow_forwardWhat is the reaction mechanism for this?arrow_forwardPredict the major products of both organic reactions. Be sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. esc esc Explanation Check 2 : + + X H₁₂O + Х ง WW E R Y qab Ccaps lock shift $ P X Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Bil T FR F18 9 G t K L Z X V B N M control opption command command T C darrow_forward
- Draw the Markovnikov product of the hydrohalogenation of this alkene. this problem. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for caps lock Explanation Check 2 W E R + X 5 HCI Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Bil Y F G H K L ZZ X C V B N M control opption command F10 F10 command 4 BA Ar Carrow_forwardI don't understand why the amide on the top left, with the R attached to one side, doesn't get substituted with OH to form a carboxylic acid. And if only one can be substituted, why did it choose the amide it chose rather than the other amide?arrow_forwardesc Draw the Markovnikov product of the hydration of this alkene. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for this problem. Explanation Check BBB + X 0 1. Hg (OAc)2, H₂O 2. Na BH 5 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Bl P 豆 28 2 28 N 9 W E R T Y A S aps lock G H K L Z X C V B N M T central H command #e commandarrow_forward
- C A student proposes the transformation below in one step of an organic synthesis. There may be one or more products missing from the right-hand side, but there are no reagents missing from the left-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. . If the student's transformation is possible, then complete the reaction by adding any missing products to the right-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. (X) This transformation can't be done in one step. + Tarrow_forwardく Predict the major products of this organic reaction. If there aren't any products, because nothing will happen, check the box under the drawing area instead. No reaction. Explanation Check OH + + ✓ 2 H₂SO 4 O xs H₂O 2 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardDraw the skeletal ("line") structure of 1,3-dihydroxy-2-pentanone. Click and drag to start drawing a structure. X Parrow_forward
- Predicting edict the major products of this organic reaction. If there aren't any products, because nothing will happen, check the box under the drawing area instead. + No reaction. Explanation Check HO Na O H xs H₂O 2 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Iarrow_forwardChoosing reagents and conditions for acetal formation or hydrolysis 0/5 A student proposes the transformation below in one step of an organic synthesis. There may be one or more products missing from the right-hand side, but there are no reagents missing from the left-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. If the student's transformation is possible, then complete the reaction by adding any missing products to the right-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + This transformation can't be done in one step. 5 I H Autumn alo 值 Ar Barrow_forwardA block of copper of mass 2.00kg(cp = 0.3851 .K) and g temperature 0°C is introduced into an insulated container in which there is 1.00molH, O(g) at 100°C and 1.00 2 atm. Note that C P = 4.184. K for liquid water, and g that A H = 2260 for water. vap g Assuming all the steam is condensed to water, and that the pressure remains constant: (a) What will be the final temperature of the system? (b) What is the heat transferred from the water to the copper? (c) What is the entropy change of the water, the copper, and the total system?arrow_forward

 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax



