
Concept explainers
a)
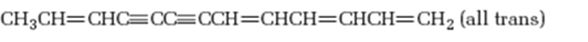
Interpretation:
The hydrocarbon shown is isolated from sun flower family. It is to be named following the IUPAC rules.
Concept introduction:
The longest carbon chain which contains the carbon-carbon triple bond is chosen. The chain is numbered from the end that gives the lowest number to the carbon in triple bond. Compounds with more than one triple bond are called diynes, triynes and so forth. Compounds containing both double bond and triple bonds are called as enynes. The chain is numbered from the end nearer to the multiple bonds, double or triple. When there is a choice in numbering the double bond is given preference and the lowest number is assigned to it.
To name:
The hydrocarbon shown, isolated from sun flower family, according to the IUPAC rules.
b)
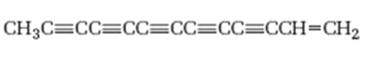
Interpretation:
The hydrocarbon shown is isolated from sun flower family. It is to be named following the IUPAC rules.
Concept introduction:
The longest carbon chain which contains the carbon-carbon triple bond is chosen. The chain is numbered from the end that gives the lowest number to the carbon in triple bond. Compounds with more than one triple bond are called diynes, triynes and so forth. Compounds containing both double bond and triple bonds are called as enynes. The chain is numbered from the end nearer to the multiple bonds, double or triple. When there is a choice in numbering the double bond is given preference and the lowest number is assigned to it.
To name:
The hydrocarbon shown, isolated from sun flower family, according to the IUPAC rules.
Trending nowThis is a popular solution!

Chapter 9 Solutions
EBK ORGANIC CHEMISTRY
- Draw the products of this reduction of a ketone with sodium borohydride. Use a dash or wedge bond to indicate the stereochemistry of substituents on asymmetric centers, where applicableIgnore any inorganic byproducts. 1) NaBH4 2) HCI/H2O Select to Drawarrow_forwardWhy do you think people who live at high altitudes are advised to add salt to water when boiling food like pasta? What mole fraction of NaCl is needed to raise the boiling point of H2O by 3˚C? Does the amount of salt added to water (typically about one teaspoon to four quarts of water) substantially change the boiling point? (Kb (H2O) = 0.51˚C/molal.)arrow_forwardpls help asaparrow_forward
- pls help asaparrow_forward9. Consider the following galvanic cell: Fe (s) | Fe(NO3)2 (aq) || Sn(NO3)2 (aq) | Sn (s) a. Write an equation for the half reactions occurring at the anode and cathode. b. Calculate the standard cell potential Show all of your work. c. Draw and label the galvanic cell, including the anode and cathode, direction of electron flow, and direction of ion migration.arrow_forwardpls help asaparrow_forward
- 11. Use the equation below to answer the following questions: 2 Al(s) + 3 Cd(NO3)2 (aq) → 2 Al(NO3)3 (aq) + 3 Cd(s) a. What is the net ionic equation for the reaction? b. Which species is a spectator ion in this reaction? Define a spectator ion. c. Identify the oxidizing agent and the reducing agent.arrow_forwardpls help asaparrow_forwardpls help asaparrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

