
a)
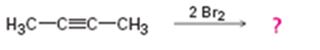
Interpretation:
Assuming that halogens add to
Concept introduction:
Alkynes when treated with one equivalent of a halogen yield a dihaloalkene as the product. They react with two equivalents of the halogens to yield a tetrahaloalkane derivative. In the first step of the addition reaction the nucleophilic attack of the π electrons of the double/triple bond in alkene/alkyne on a halogen results in the formation of a cyclic halonium ion with the simultaneous elimination of a halide ion. In the second step the halide ion attacks the cyclic halonium ion to yield the product.
To propose:
A mechanism and to predict the product(s) expected for the reaction in which two equivalents of Br2 adds to 2-butyne assuming that bromine adds to alkynes in the same manner as they add to alkenes.
b)
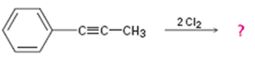
Interpretation:
Assuming that halogens add to alkynes in the same manner as they add to alkenes, a mechanism is to be proposed and the product(s) expected for the reaction in which two equivalents of Cl2 adds to 1-phenylpropyne is/are to be predicted.
Concept introduction:
Alkynes when treated with one equivalent of a halogen yield a dihaloalkene as the product. They react with two equivalents of the reagents to yield a tetrahaloalkane as the product. In the first step of the addition reaction, the nucleophilic attack of the π electrons of the double/triple bond in alkene/alkyne on a halogen results in the formation of a cyclic halonium ion with the simultaneous elimination of a halide ion. In the second step the halide ion attacks the cyclic halonium ion to yield the product.
To propose:
A mechanism and to predict the product(s) expected for the reaction in which two equivalents of Cl2 adds to 1-phenylpropyne assuming that chlorine adds to alkynes in the same manner as they add to alkenes.
c)
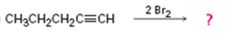
Interpretation:
Assuming that halogens add to alkynes in the same manner as they add to alkenes, a mechanism is to be proposed and the product(s) expected for the reaction in which two equivalents of Br2 adds to 1-pentyne is/are to be predicted.
Concept introduction:
Alkynes when treated with one equivalent of a halogen yield a dihaloalkene as the product. They react with two equivalents of the reagents to yield a tetrahaloalkane as the product. In the first step of the addition reaction the nucleophilic attack of the π electrons of the double/triple bond in alkene/alkyne on a halogen results in the formation of a cyclic halonium ion with the simultaneous elimination of a halide ion. In the second step the halide ion attacks the cyclic halonium ion to yield the product.
To propose:
A mechanism and to predict the product(s) expected for the reaction in which two equivalents of Br2 adds to 1-pentyne assuming that bromine adds to alkynes in the same manner as they add to alkenes.
Trending nowThis is a popular solution!

Chapter 9 Solutions
Student Value Bundle: Organic Chemistry, + OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card (NEW!!)
- In an experiment, the viscosity of water was measured at different temperatures and the table was constructed from the data obtained. a) Calculate the activation energy of viscous flow (kJ/mol). b) Calculate the viscosity at 30°C. T/°C 0 20 40 60 80 η/cpoise 1,972 1,005 0,656 0,469 0,356arrow_forwardDon't used Ai solutionarrow_forwardLet's see if you caught the essentials of the animation. What is the valence value of carbon? a) 4 b) 2 c) 8 d) 6arrow_forward
- A laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).arrow_forwardA laser emits a line at 632.8 nm. If the cavity is 12 cm long, how many modes oscillate in the cavity? How long does it take for the radiation to travel the entire cavity? What is the frequency difference between 2 consecutive modes?(refractive index of the medium n = 1).arrow_forwardThe number of microstates corresponding to each macrostate is given by N. The dominant macrostate or configuration of a system is the macrostate with the greatest weight W. Are both statements correct?arrow_forward
- For the single step reaction: A + B → 2C + 25 kJ If the activation energy for this reaction is 35.8 kJ, sketch an energy vs. reaction coordinate diagram for this reaction. Be sure to label the following on your diagram: each of the axes, reactant compounds and product compounds, enthalpy of reaction, activation energy of the forward reaction with the correct value, activation energy of the backwards reaction with the correct value and the transition state. In the same sketch you drew, after the addition of a homogeneous catalyst, show how it would change the graph. Label any new line "catalyst" and label any new activation energy.arrow_forwardHow many grams of C are combined with 3.75 ✕ 1023 atoms of H in the compound C5H12?arrow_forwarde. f. CH3O. יון Br NaOCH3 OCH 3 Br H₂Oarrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



