
PKG ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259963667
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 9.78P
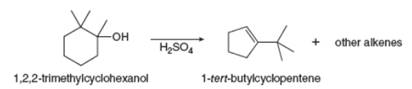
Dehydration of
shows how this alkene is formed. (b) Draw other
dehydration. At least one must contain a five-membered ring.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Name the following molecules using IUPAC.
Identify each alcohol in the molecule as either primary, secondary, or tertiary.
Draw the mechanism for the grignard reagent reactant.
Chapter 9 Solutions
PKG ORGANIC CHEMISTRY
Ch. 9 - Problem 9.1 Label each ether and alcohol in...Ch. 9 - Give the IUPAC name for each compound.Ch. 9 - Problem 9.3 Give the structure corresponding to...Ch. 9 - Name each of the following ethers.Ch. 9 - Name each epoxide.
a. (two ways) b. c. (two...Ch. 9 - Problem 9.6 Rank the following compounds in order...Ch. 9 - Which mechanism is favored by the use of crown...Ch. 9 - Problem 9.8 Draw the organic product of each...Ch. 9 - Prob. 9.9PCh. 9 - Problem 9.10 Draw the products of each reaction.
...
Ch. 9 - Problem 9.11 Draw the products formed when each...Ch. 9 - Prob. 9.12PCh. 9 - Problem 9.13 Draw the structure of each...Ch. 9 - What other alkene is also formed along with Y in...Ch. 9 - Prob. 9.15PCh. 9 - Explain why two substitution products are formed...Ch. 9 - Draw the products of each reaction. a. b. c.Ch. 9 - Problem 9.18 Draw the products of each reaction,...Ch. 9 - Problem 9.19 What is the major product formed...Ch. 9 - Prob. 9.20PCh. 9 - Prob. 9.21PCh. 9 - Problem 9.22 Draw the organic products formed in...Ch. 9 - Problem 9.23 Draw two steps to convert into each...Ch. 9 - Prob. 9.24PCh. 9 - Problem 9.25 Draw the products of each reaction,...Ch. 9 - Draw the products formed when (S)-butan-2-ol is...Ch. 9 - Draw the product formed when (CH3)2CHOH is treated...Ch. 9 - What alkyl halides are formed when each ether is...Ch. 9 - Explain why the treatment of anisole with HBr...Ch. 9 - Name each thiol.
a. b.
Ch. 9 - Draw the product of each reaction. ac b.d.Ch. 9 - Give the IUPAC name for each sulfide.
a. b.
Ch. 9 - Draw the product of each reaction.
a. b.
Ch. 9 - Prob. 9.34PCh. 9 - The cis and trans isomers of 2, 3-dimethyloxirane...Ch. 9 - Problem 9.36 Draw the product of each...Ch. 9 - 9.37 Name each compound depicted in the...Ch. 9 - Answer each question using the ball-and-stick...Ch. 9 - Prob. 9.39PCh. 9 - 9.40 Give IUPAC name for each...Ch. 9 - Prob. 9.41PCh. 9 - Prob. 9.42PCh. 9 - Prob. 9.43PCh. 9 - 9.44 Why is the boiling point of higher than...Ch. 9 - 9.45 Draw the organic product(s) formed when is...Ch. 9 - 9.46 What alkenes are formed when each alcohol is...Ch. 9 - Prob. 9.47PCh. 9 - 9.48 Draw the products of each reaction and...Ch. 9 - 9.49 Draw the product of the following reaction,...Ch. 9 - Prob. 9.50PCh. 9 - Prob. 9.51PCh. 9 - 9.52 Draw a stepwise mechanism for the following...Ch. 9 - 9.53 Although alcohol V gives a single alkene W...Ch. 9 - Prob. 9.54PCh. 9 - Prob. 9.55PCh. 9 - Prob. 9.56PCh. 9 - 9.57 Draw a stepwise, detailed mechanism for the...Ch. 9 - Prob. 9.58PCh. 9 - 9.59 Draw two different routes to each of the...Ch. 9 - Prob. 9.60PCh. 9 -
9.61 Draw the products formed when each ether is...Ch. 9 - 9.62 Draw a stepwise mechanism for each...Ch. 9 - Draw a stepwise, detailed mechanism for the...Ch. 9 - Prob. 9.64PCh. 9 - Draw the products of each reaction. a.c. b.d.Ch. 9 - When each halohydrin is treated with, a product of...Ch. 9 - Prob. 9.67PCh. 9 - Prob. 9.68PCh. 9 - Prob. 9.69PCh. 9 - Prob. 9.70PCh. 9 - Prepare each compound from cyclopentanol. More...Ch. 9 - 9.72 Identify the reagents (a–h) needed to carry...Ch. 9 - Prob. 9.73PCh. 9 - 9.74 Treatment of with affords compound A and ....Ch. 9 - Prob. 9.75PCh. 9 - Prob. 9.76PCh. 9 - 9.77 Draw a stepwise, detailed mechanism for the...Ch. 9 - 9.78 Dehydration of with affords
as a minor...Ch. 9 - Prob. 9.79PCh. 9 - 9.80 Draw a stepwise mechanism for the following...Ch. 9 -
9.81 Aziridines are heterocycles that contain an...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Give the IUPAC name for each compound.
Organic Chemistry
Some people consider Pasteur or Koch to be the Father of Microbiology, rather than Leeuwenhoek. Why might they ...
Microbiology with Diseases by Body System (5th Edition)
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties (2nd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me figure out the mechanism with arrows of the following reactionarrow_forwardOrganic Functional Groups Predicting the reactants or products of acetal hydrolysis termine the structures of the missing organic molecules in the following reaction: H* H* + H₂O Y ☑ Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X, Y, and Z. You may draw that you like, so long as they aren't touching. Molecule X shows up in multiple steps, but you only have to draw its structure Explanation Check @2 W Click and drag to start drawing a structure. #4 # 3 LU E % 67 olo 5 66 R T Y & 7 AcGraw Hill LLC. All Rights R Xarrow_forward8. (16 pts) Provide the stepwise mechanism for the synthesis of the following compound via an enaminearrow_forward
- Draw the titration curve of (i) weak acid vs. strong base; (ii) weak acid vs. weakbase; (iii) diprotic acid with strong base (iii) triprotic acid with strong base.arrow_forwardComplete the reaction in the drawing area below by adding the major products to the right-hand side. If there won't be any products, because nothing will happen under these reaction conditions, check the box under the drawing area instead. Note: if the products contain one or more pairs of enantiomers, don't worry about drawing each enantiomer with dash and wedge bonds. Just draw one molecule to represent each pair of enantiomers, using line bonds at the chiral center. More... No reaction. my ㄖˋ + 1. Na O Me Click and drag to start drawing a structure. 2. H +arrow_forwardPredict the intermediate 1 and final product 2 of this organic reaction: NaOMe H+ + 1 2 H H work up You can draw 1 and 2 in any arrangement you like. Note: if either 1 or 2 consists of a pair of enantiomers, just draw one structure using line bonds instead of 3D (dash and wedge) bonds at the chiral center. Click and drag to start drawing a structure. X $ dmarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License