
(a)
Interpretation: The product of the given reaction is to be drawn including stereochemistry if appropriate.
Concept introduction: Alkyl tosylates undergo elimination reaction when they are allowed to react with strong nucleophilic base. The mechanism of the elimination reaction is
Answer to Problem 9.69P
The product of the given reaction is,
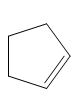
Figure 2
Explanation of Solution
The given reaction involves treatment of alkyl tosylate with
Alkyl tosylates undergo elimination reaction when they are allowed to react with strong nucleophilic base. The mechanism of the reaction is
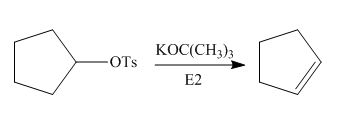
Figure 1
Thus, the product of the given reaction is,

Figure 2
The product of the given reaction is drawn in Figure 2.
(b)
Interpretation: The product of the given reaction is to be drawn including stereochemistry if appropriate.
Concept introduction: The reaction of alcohols with halogen acids
Answer to Problem 9.69P
The product of the given reaction is,
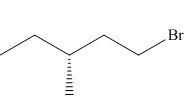
Figure 4
Explanation of Solution
The given reaction involves treatment of an alcohol with
The reaction of alcohols with halogen acids
The corresponding reaction is shown below.
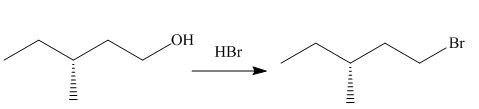
Figure 3
Thus, the product of the given reaction is,

Figure 4
The product of the given reaction is drawn in Figure 4.
(c)
Interpretation: The product of the given reaction is to be drawn including stereochemistry if appropriate.
Concept introduction: Thiols react with
Answer to Problem 9.69P
The product of the given reaction is,
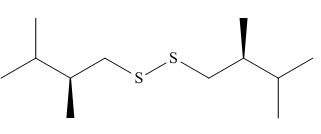
Figure 6
Explanation of Solution
The given reaction involves treatment of thiol with
Thiols react with
The corresponding reaction is shown below.
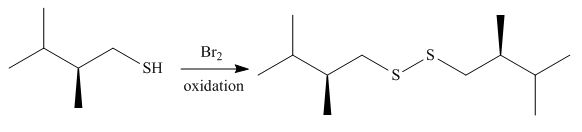
Figure 5
Thus, the product of the given reaction is,

Figure 6
The product of the given reaction is drawn in Figure 6.
(d)
Interpretation: The product of the given reaction is to be drawn including stereochemistry if appropriate.
Concept introduction: The
Answer to Problem 9.69P
The product of the given reaction is,
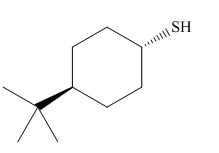
Figure 8
Explanation of Solution
The given reaction involves treatment of alkyl tosylate with
The
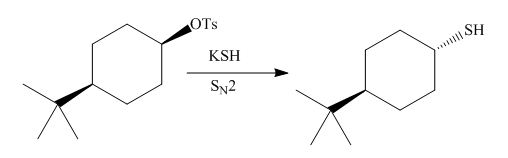
Figure 7
Thus, the product of the given reaction is,

Figure 8
The product of the given reaction is drawn in Figure 8.
(e)
Interpretation: The product of the given reaction is to be drawn including stereochemistry if appropriate.
Concept introduction: Alkyl bromides are obtained by the reaction of
Answer to Problem 9.69P
The product of the given reaction is,
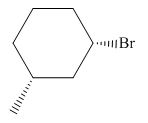
Figure 10
Explanation of Solution
The given reaction involves treatment of secondary alcohol with
Alkyl bromides are obtained by the reaction of
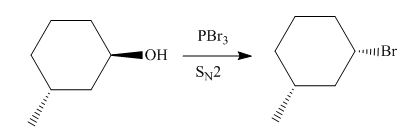
Figure 9
Thus, the product of the given reaction is,

Figure 10
The product of the given reaction is drawn in Figure 10.
(f)
Interpretation: The product of the given reaction is to be drawn including stereochemistry if appropriate.
Concept introduction: Alcohols are converted into alkyl tosylates by treatment with
Answer to Problem 9.69P
The product of the given reaction is,
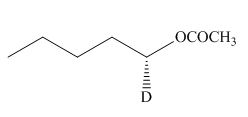
Figure 12
Explanation of Solution
The given reaction involves treatment of an alcohol with
Alcohols are converted into alkyl tosylates by treatment with
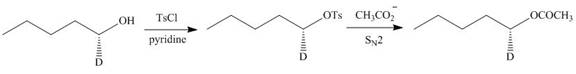 Figure 11
Figure 11
Thus, the product of the given reaction is,

Figure 12
The product of the given reaction is drawn in Figure 12.
(g)
Interpretation: The product of the given reaction is to be drawn including stereochemistry if appropriate.
Concept introduction: The opening of an
Answer to Problem 9.69P
The product of the given reaction is,
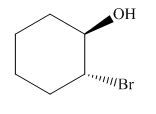
Figure 14
Explanation of Solution
The give reaction involves treatment of an epoxide with halogen acid
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
The corresponding reaction is shown below.
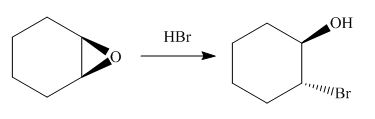
Figure 13
Thus, the product of the given reaction is,

Figure 14
The product of the given reaction is drawn in Figure 14.
(h)
Interpretation: The product of the given reaction is to be drawn including stereochemistry if appropriate.
Concept introduction: The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
Answer to Problem 9.69P
The product of the given reaction is,
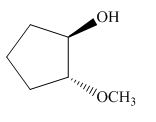
Figure 16
Explanation of Solution
The give reaction involves treatment of an epoxide with
The opening of an epoxide/ethylene oxide ring is regioselective either it takes place with a strong nucleophile
The corresponding reaction is shown below.
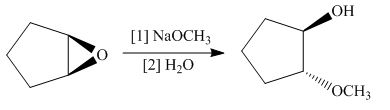
Figure 15
Thus, the product of the given reaction is,

Figure 16
The product of the given reaction is drawn in Figure 16.
(i)
Interpretation: The product of the given reaction is to be drawn including stereochemistry if appropriate.
Concept introduction: Ethers are the most common organic products of nucleophilic substitution reaction. They are prepared from alkyl halides and strong nucleophiles. The reaction proceeds through
Answer to Problem 9.69P
The product of the given reaction is,
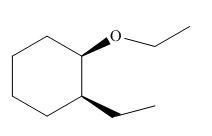
Figure 18
Explanation of Solution
The given reaction involves treatment of a
The alkoxide salts are prepared from alcohols through the Bronsted-Lowry acid-base reaction. In this reaction,
The obtained alkoxide from this reaction contains
The corresponding reaction is shown below.
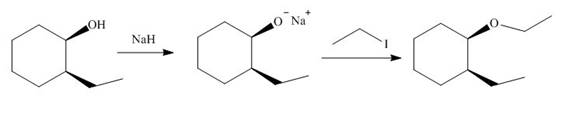
Figure 17
Thus, the product of the given reaction is,
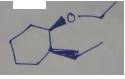
Figure 18
The product of the given reaction is drawn in Figure 18.
(j)
Interpretation: The product of the given reaction is to be drawn including stereochemistry if appropriate.
Concept introduction: Ethers react with strong acids, (only
In this reaction, both
Answer to Problem 9.69P
The product of the given reaction is,
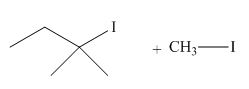
Figure 20
Explanation of Solution
The given reaction involves treatment of an ether with two equivalents of
Ethers react with strong acids, (only
In this reaction, both
The corresponding reaction is shown below.
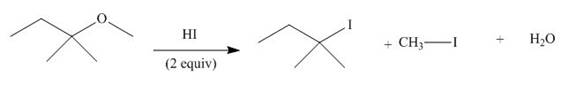
Figure 19
Thus, the product of the given reaction is,

Figure 20
The product of the given reaction is drawn in Figure 20.
(k)
Interpretation: The product of the given reaction is to be drawn including stereochemistry if appropriate.
Concept introduction: Sulfides are prepared from thiols by the successive treatment of sodium hydride (a good base), and an alkyl halide. The mechanism of the reaction is
Answer to Problem 9.69P
The product of the given reaction is,
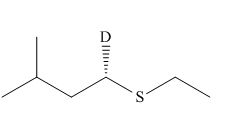
Figure 22
Explanation of Solution
The given reaction involves treatment of an alkyl halide with
The corresponding reaction is shown below.
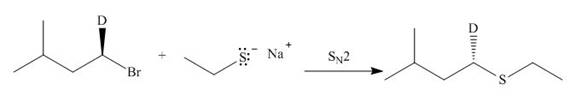
Figure 21
Thus, the product of the given reaction is,

Figure 22
The product of the given reaction is drawn in Figure 22.
(l)
Interpretation: The product of the given reaction is to be drawn including stereochemistry if appropriate.
Concept introduction: Sulfide involves a nucleophilic sulfur atom. It reacts rapidly with unhindered alkyl halide to form corresponding sulfonium ion. The mechanism of the reaction is
Answer to Problem 9.69P
The product of the given reaction is,

Figure 24
Explanation of Solution
The given reaction involves treatment of sulfide with an unhindered alkyl halide.
Sulfide involves a nucleophilic sulfur atom. It reacts rapidly with unhindered alkyl halide to form corresponding sulfonium ion. The mechanism of the reaction is
The corresponding reaction is shown below.
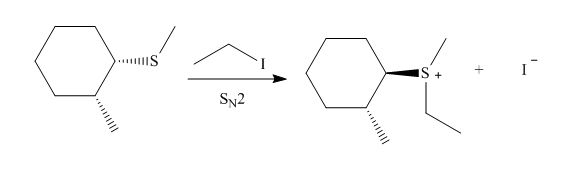
Figure 23
Thus, the product of the given reaction is,
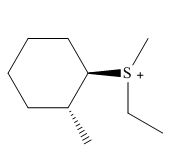
Figure 24
The product of the given reaction is drawn in Figure 24.
Want to see more full solutions like this?
Chapter 9 Solutions
PKG ORGANIC CHEMISTRY
- Assign the functional group bands on the IR spectra.arrow_forwardFind the pH of a 0.120 M solution of HNO2. Find the pH ignoring activity effects (i.e., the normal way). Find the pH in a solution of 0.050 M NaCl, including activityarrow_forwardPlease help me answer these three questions. Required info should be in data table.arrow_forward
- Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forward
- Order the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





