
Concept explainers
(a)
Interpretation:
It is to be determined whether the given
Concept introduction:
Competing reactions can take place in kinetic or
Reactions that tend to take place under thermodynamic control are the ones in which a more stable but not necessarily the major product is formed.
Reversible reactions tend to take place under thermodynamic control, while irreversible reactions tend to take place under kinetic control.
Reactions like
The charge stability decides if the products are more stable than the reactants or vice versa. A reaction is irreversible if it’s
Answer to Problem 9.72P
The given reaction is irreversible.
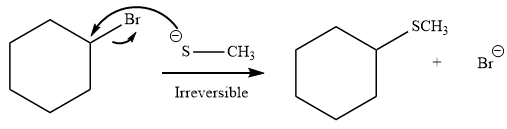
Explanation of Solution
The given reaction is:
It is mentioned that the reaction would follow the
The species
As the reaction is irreversible it would take place under kinetic control.
The charge stability decides if the products are more stable than the reactants or vice versa.
(b)
Interpretation:
It is to be determined whether the given
Concept introduction:
Competing reactions can take place in kinetic or thermodynamic control. Reactions that tend to take place under the kinetic control are the ones in which the major product is the one that forms the fastest.
Reactions that tend to take place under thermodynamic control are the ones in which a more stable but not necessarily the major product is formed.
Reversible reactions tend to take place under thermodynamic control, while irreversible reactions tend to take place under kinetic control.
Reactions like
The charge stability decides if the products are more stable than the reactants or vice versa. A reaction is irreversible if it’s
Answer to Problem 9.72P
The given reaction is reversible.
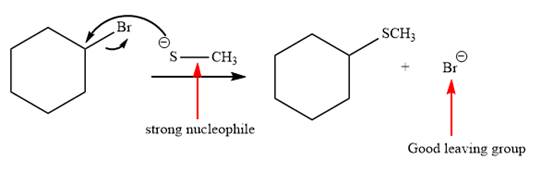
Explanation of Solution
The given reaction is:
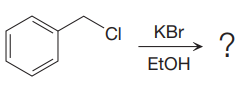
It is mentioned that the reaction would follow the
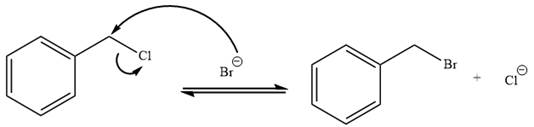
The charge stability decides if the products are more stable than the reactants or vice versa. The charged species on the left side is
This makes the reaction faster in the reverse direction than in the forward direction under standard conditions. Thus, this reaction is reversible.
The charge stability decides if the products are more stable than the reactants or vice versa.
(c)
Interpretation:
It is to be determined whether the given
Concept introduction:
Competing reactions can take place in kinetic or thermodynamic control. Reactions that tend to take place under the kinetic control are the ones in which the major product is the one that forms the fastest.
Reactions that tend to take place under thermodynamic control are the ones in which a more stable but not necessarily the major product is formed.
Reversible reactions tend to take place under thermodynamic control, while irreversible reactions tend to take place under kinetic control.
Reactions like
The charge stability decides if the products are more stable than the reactants or vice versa. A reaction is irreversible if it’s
Answer to Problem 9.72P
The given reaction is irreversible.
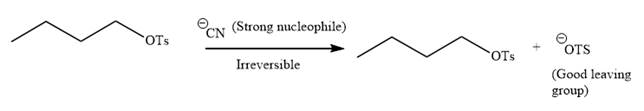
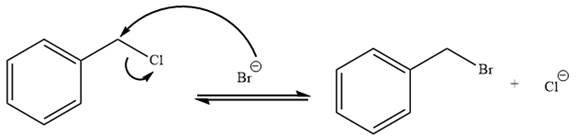
Explanation of Solution
The given reaction is:
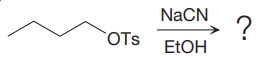
It is mentioned that the reaction would follow the
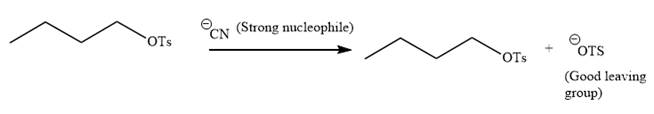
In the above reaction,
The charge stability decides if the products are more stable than the reactants or vice versa. The charged species on the left side is
Thus,
As the reaction is irreversible it would take place under kinetic control.
The charge stability decides if the products are more stable than the reactants or vice versa.
Want to see more full solutions like this?
Chapter 9 Solutions
Get Ready for Organic Chemistry
- H I T H HH H -H C. H- Identify and select all structures below that represent a constitutional isomer(s) of the compound shown above. H- H CIH H H H HHHH H H 0 ·H H– 冊 CH CHI HH C- H- H H- H H A. H H C H H- -H HH H B. H- -H D. H H H H • H -H E. -H H H HICH T HHH F. H-arrow_forwardPolylactic acid (shown below) is a biodegradable polymer used for food packaging. Identify the monomer(s) used in the production of this polymer using a condensation process.arrow_forwardDraw the product of the reaction shown below. Ignore small byproducts that would evaporate pleasearrow_forward
- Poly(ethylene adipate) is a biodegradable polyester (shown below). Identify the type of polymerization process used in the production of this polymer.arrow_forwardPolymers may be composed of thousands of monomers. draw two repeat units(dimer) of the polymer formed in this reaction. assume there are hydrogen atoms on the two ends of the dimer. ignore inorganic byproducts pleasearrow_forwardDraw the product of the reaction shown below. Use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers, Ignore inorganic byproductsarrow_forward
- Draw the product of this reaction please. Ignore inorganic byproductsarrow_forwardOne of the pi molecular orbitals of 1,3-butadiene (CH2=CHCH=CH2) is shown below. Please identify the number of nodal planes perpendicular to the bonding axisarrow_forwardDraw the monomers required to synthesize this condensation polymer please.arrow_forward
- Provide the correct systematic name for the compound shown here. Please take into account the keyboard options belowarrow_forwardcurved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s)arrow_forwardIdentify the 'cartoon' drawing of the acceptor orbital in the first mechanistic step of an electrophilic addition reaction of butadiene with HBr. Pleasearrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

