
(Tribology) Why you change your motor oil? One of the major reasons for engine-oil degradation is the oxidation of the motor oil. To retard the degradation process, most oils contain an antioxidant [see Ind. Eng. Chem. 26, 902 (1987)]. Without an inhibitor to oxidation present, the suggested

where I2 is an initiator and RH is the hydrocarbon in the oil.
When an antioxidant is added to retard degradation at low temperatures, the following additional termination steps occur:
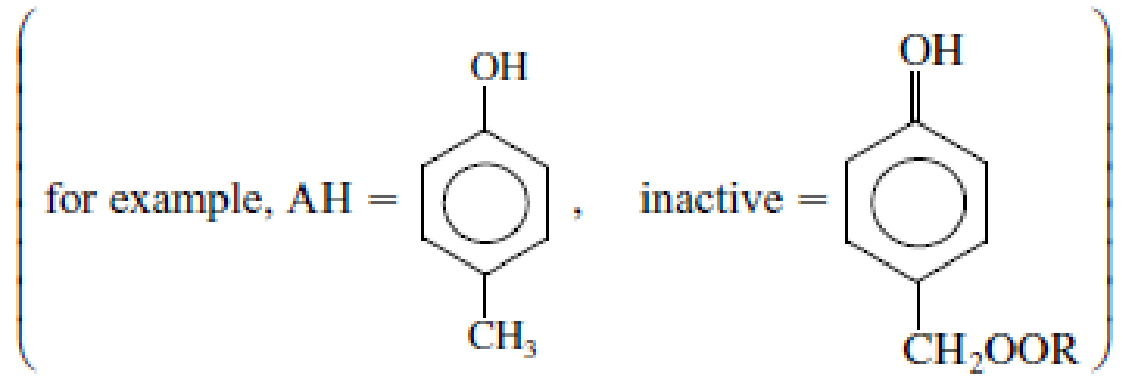
- (a) Derive a rate law for the degradation of the motor oil in the absence of an antioxidant at low temperatures.
- (b) Derive a rate law for the rate of degradation of the motor oil in the presence of an antioxidant for low temperatures.
- (c) How would your answer to part (a) change if the radicals I· were produced at a constant rate in the engine and then found their way into the oil?
- (d) Sketch a reaction pathway diagram for both high and low temperatures, with and without antioxidant.
- (e) See the open-ended problem G.2 in Appendix G and on the CRE Web site for more on this problem.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
ELEMENTS OF CHEM. REACTION ENGR
Additional Engineering Textbook Solutions
Java How to Program, Early Objects (11th Edition) (Deitel: How to Program)
Modern Database Management
Degarmo's Materials And Processes In Manufacturing
Mechanics of Materials (10th Edition)
Starting Out With Visual Basic (8th Edition)
Concepts Of Programming Languages
- Water at 60° F is being pumped from a stream to a reservoir whose surface is 210 ft above the pump. The pipe from the pump to the reservoir is an 8-in Schedule 40 steel pipe 2500 ft long. The pressure at the pump inlet is - 2,36 psig. If 4.00 ft³/s is being pumped, a). Compute the pressure at the outlet of the pump. Answer: 0,997 MPa b). Compute the power delivered by the pump to the water. Answer: 151 hp Consider the friction loss in the discharged line, but neglect other lossesarrow_forward1. Consider a mixture of 2.5.0% ethane, 2.0% butane, and 1.7% n-pentane by volume.a. Estimate the LFL and UFL of the mixture. Is it flammable?b. Estimate the LOC for this mixture.arrow_forwardEstimate the LFL and UFL for propylene using Equations 6-10 and 6-11 in the textbook,and compare these to the experimental values given in the table in Appendix B.arrow_forward
- 1. Determine the minimum compression ratio required to raise the temperature of air overhexane to its AIT. Assume an initial temperature of 20°C.2. Ethanol is kept in a storage vessel that is vented with air (at 25°C and 1 atm). Is theequilibrium mixture of vapor above the liquid and air flammable? What if the liquid isacetone instead?arrow_forwardHydrogenation of Ethylbenzene to Styrene Reaction: C₈H₁₀ → C₈H₈ + H₂ΔHᵣ°(300°C) = -124 kJ/mol (exact value unknown) Process Description: The basis is 1000 kg/h of separated styrene. The reaction conversion rate is 35%. The temperature increase in heat exchanger 2 is adiabatic. A fresh stream of pure ethylbenzene (25°C) enters a mixing vessel, where it is combined with a recycle stream (from the distillation column, as explained later), which also consists of pure ethylbenzene at 25°C. After mixing, the stream is sent to a heat exchanger (HX1), where the mixture is heated to 200°C. Next, the mixture enters an adiabatic heat exchanger (HX2), where it is further heated to 300°C by adding steam (at 350°C). This steam is used to prevent side reactions and carbon deposition in the reactor. The heated mixture is then fed into the reactor, where the reaction takes place with a conversion rate of 35%. As a result, the mixture cools down to 260°C. The resulting mixture is then sent to HX4, where…arrow_forwardChemical Engineering Questionarrow_forward
- 4.16 aarrow_forward8. The thermal decomposition of nitric oxide at elevated temperatures 2NO → N₂+02 has been studied in a batch reactor where at temperatures below 2000K the rate expression that applies to low conversions is: r = kCm05 Co At high conversions, or when the initial mixture contains a high concentration of O2 the rate expression is given by: r = k' Cм0.5 C15C0,5 To explain these kinetics the following chain reaction mechanism has been proposed: Initiation: Propagation: 2NON₂O +0 k₂ E1=272.0 kJ/mol 0+ NO O₂+ N E₂-161.0 kJ/mol N+NO N₂+0 E3-1.4 kJ/mol K4 20+ MO₂+M E4=14.0 kJ/mol ks Termination: where M is any molecule capable of the energy transfer necessary to stabilize the oxygen molecule. Once appreciable amounts of O2 are present in the reaction mixture, the initiation reaction that is the primary source of atomic oxygen is no longer the first reaction. Instead, the following reaction begins to dominate the chain initiation process: Initiation (high O2): ks NO +0₂ NO₂+0 E5=198.0 kJ/mol a.…arrow_forward2:41 2) If the number-average degree of polymerization for styrene obtained by the bulk polymerization at 25°C is 5,000, what would be the number-average degree of polymerization if conducted in a 10% solution in toluene (900g of toluene per 100 g of styrene) under otherwise identical conditions? State any assumptions that are needed. (see Table 2-4). Table 2-4 Representative Values of Chain-Transfer Constants Monomer Styrene Chain-Transfer Agent T (°C) C x 104 Styrene 25 bas 0.279 * 50 0.35-0.78 Polystyrene 50 1.9-16.6 Benzoyl peroxide 50 0.13 Toluene 60 0.125 Methyl methacrylate Methyl methacrylate 30 0.117 70 0.2 Poly(methyl methacrylate) 50 0.22-1000 Benzoyl peroxide 50 0.01 Toluene 40 0.170 3) 2 3) Methyl methacrylate is copolymerized with 2-methylbenzyl methacrylate (M₁) in 1,4- dioxane at 60°C using AIBN as the free-radical initiator. (a) Draw the repeating unit of poly(2-methylbenzyl methacrylate). (b) From the data given in the table below, estimate the reactivity ratios of…arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





