
Concept explainers
(a)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(a)
Explanation of Solution
Electronic configuration of
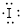
(b)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(b)
Explanation of Solution
Electronic configuration of
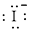
(c)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(c)
Explanation of Solution
Electronic configuration of
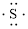
(d)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(d)
Explanation of Solution
Electronic configuration of
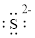
(e)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(e)
Explanation of Solution
Electronic configuration of
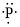
(f)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(f)
Explanation of Solution
Electronic configuration of
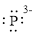
(g)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(g)
Explanation of Solution
Electronic configuration of
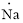
(h)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(h)
Explanation of Solution
Electronic configuration of
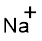
(i)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(i)
Explanation of Solution
Electronic configuration of
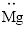
(j)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(j)
Explanation of Solution
Electronic configuration of
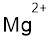
(k)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(k)
Explanation of Solution
Electronic configuration of
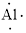
(l)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(l)
Explanation of Solution
Electronic configuration of
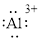
(m)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(m)
Explanation of Solution
Electronic configuration of
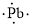
(n)
Interpretation:
Lewis dot symbol for
Concept Introduction:
Lewis dot symbol represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(n)
Explanation of Solution
Electronic configuration of
Want to see more full solutions like this?
Chapter 9 Solutions
General Chemistry
- Iarrow_forwardDraw the Markovnikov product of the hydrobromination of this alkene. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for this problem. + Explanation Check 1 X E 4 1 1 1 1 1 HBr Click and drag to start drawing a structure. 80 LE #3 @ 2 $4 0 I அ2 % 85 F * K M ? BH 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center & 6 27 FG F10 8 9 R T Y U D F G H P J K L Z X C V B N M Q W A S H option command H command optiarrow_forwardBe sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. Predict the major products of the following reaction. Explanation Q F1 A Check F2 @ 2 # 3 + X 80 F3 W E S D $ 4 I O H. H₂ 2 R Pt % 05 LL ee F6 F5 T <6 G Click and drag to start drawing a structure. 27 & A 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Acce Y U H DII 8 9 F10 4 J K L Z X C V B N M T H option command F11 P H commandarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the arrows to draw the intermediate and product in this reaction or mechanistic step(s). Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore inorganic byproducts. H :0: CH3 O: OH Q CH3OH2+ Draw Intermediate protonation CH3OH CH3OH nucleophilic addition H Draw Intermediate deprotonation :0: H3C CH3OH2* protonation H 0: H CH3 H.arrow_forwardPredicting the reactants or products of hemiacetal and acetal formation uentify the missing organic reactants in the following reaction: H+ X+Y OH H+ за Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Explanation Check Click and drag to start drawing a structure. ? olo 18 Ar © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardcan someone please answer thisarrow_forward
- Please, please help me figure out the the moles, molarity and Ksp column. Step by step details because I've came up with about three different number and have no idea what I'm doing wrong.arrow_forwardwhat reagents are used to get this product from this reactant? Br OCH3arrow_forwardcan someone answer this pleasearrow_forward
- can someone do the reaction mechanism for this reaction and draw the molecules for Q2 and q3arrow_forwardIn this question, the product of the aldol condensation is shown. What would be the reactants for this product? Please provide a detailed explanation, as well as a drawing showing how the reactants will react to produce the product.arrow_forward7. Propene undergoes a hydration reaction with water in the presence of an acid. a. There are two possible products for this reaction, both with the formula C,H,O. Show their structural formulas and names. (A1, B2) SCH4UR Name: (answer for part a. here!) VER 3 2021-2022 b. Which of the two products do you predict will form. Explain your choice using details from your learning. (B3)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





