
Concept explainers
(a)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in
atomic number and increase by four in mass number. - Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
Of the radioisotopes listed in Table-9.5, the majority decay by gamma radiation.Thus, the statement is false.
Explanation of Solution
Table-9.5 contains the lists of some radioactive isotopes which are used in medical imaging. As per the data provided in table, there are maximum numbers of radioactive isotopes that emits gamma radiation.
(b)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
Isotopes that decay by alpha emission are rarely if ever used in nuclear imaging because alpha emitters are rare.Thus, the statement is true.
Explanation of Solution
Table-9.5 with the list of radioactive isotopes used in medical imaging contains no radioactive isotope that emits alpha particles. There are very rare isotopes useful in medical like radium-223 for treatment of cancer.
(c)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
Gamma emitters are so widely used in medical imaging because gamma radiation is penetrating and, therefore, can easily be measured by radiation detectors outside the body.Thus, the statement is true.
Explanation of Solution
Gamma emitter are widely used because of gamma particles had no mass and charge and they can easily penetrate in body.
(d)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
When selenium-75 deays by electron capture and gamma emission, the new nucleus formed is arsenic-75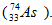 ).Thus, the statement is false.
).Thus, the statement is false.
Explanation of Solution
When selenium-75 decays by electron capture, a new nuclues formed should contain 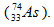
(e)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
When iodine-131 decays by beta and gamma emission, the new nucleus formed is xenon-131 (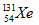 ).Thus, the statement is true.
).Thus, the statement is true.
Explanation of Solution
During the decay of iodine-131 by beta and gamma emission, the new nucleus formed contains one number more in atomic mass. This new nucleus is Xenon-131 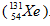
(f)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
In positron emission tomography (a PET scan), the detectors counts the numbers of gamma particle emitted by a tagged material and the location within the body where the tagged materail accumulates. Thus, the statement is false.
Explanation of Solution
The radiation contains particles with energy which are emitted by radioactive isotopes. This energy is useful to trace the particle within the body during its medical use. The Positron Tomography Scan contains Positrons which has very short live and they collide with electron and forms gamma rays. PET scanner capture the energy of the particles and it does not count the numbers of positrons.
(g)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
The use of 18-fluorodeoxyglucose (FDG) in PET scans of the brain depends on the fact that FDG behaves in the body as does glucose.Thus, the statement is true.
Explanation of Solution
The use of 18-fluorodeoxyglucose is as glucose in the body because the six carbons of glucose are replaced by fluorine-18 and it can easily cross the Blood-brain barrier.
(h)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
A goal of raditaion therapy is to destroy pathological cells and tissues without at the same time damaging normal cells and tissues.Thus, the statement is true.
Explanation of Solution
The radiation therapy has a goal to just destroy pathological cells and tissues but inevitably it also damages healthy and normal cells and tissue that surrounds damaged tissues.
(i)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
In external beam radition, radiation from an external source is directed at a tissue from outside the body.Thus, the statement is false.
Explanation of Solution
In external beam radiation, a high energy beam is used to target the tumor cells from outside the body. It destroys the cells which are available inside the body and not the surface of body.
(j)
Interpretation:
If the given statement is true or false should be determined.
Concept Introduction:
Isotopes which are having balance numbers of protons and neutrons are stable, but the serious imbalance can lead to nuclear reaction. Radioactive isotopes emit alpha, beta, positron, gamma particles due to nuclear reactions.
- Alpha emission is an emission of helium nucleus (2 Protons and 2 neutrons) from the elements to stabilize the nucleus. This emission results into decrease by two in atomic number and increase by four in mass number.
- Beta emission is an emission of beta particle as electron from nucleus of molecule. This emission results into increase in atomic number and no change in mass number.
- Positron emission is an emission in electron with positive charge is emitted by nucleus of molecule. This emission results into decrease by one number in atomic number and no change in mass number.
- Gamma radiation is only access energy from nucleus is emitted to stabilize nucleus of molecule. This emission results into no change in mass number and atomic number.
These emitted particles are useful in diagnosis and treatment of diseases as a nuclear medicine. They act as invaluable tool in fieled of science. These nuclear chemistry is utilized in medicla imaging like PET (Positron emission tomography), X-rays, MRI (Magnetic resonance Imaging), CT scan etc.
Answer to Problem 9.50P
In internal beam radiation, a radioactive material is implnated in a target tissue to destroy cells in the target tissue without doing appreciable damage to surrounding normla tissues.Thus, the statement is true.
Explanation of Solution
In internal beam radiation therapy, a radioactive material is implanted into body which is located very near to the tumor cells or tissues. This type of radiation does not make more damage to the surrounding tissues or cells.
Want to see more full solutions like this?
Chapter 9 Solutions
Introduction to General, Organic and Biochemistry
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: N2O4 (g) 2NO2 (g) AG⁰ = 5.4 kJ Now suppose a reaction vessel is filled with 1.68 atm of dinitrogen tetroxide (N204) at 148. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N2O4 tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO2? In other words, if you said the pressure of N2O4 will tend to rise, can that be changed to a tendency to fall by adding NO2? Similarly, if you said the pressure of N2O4 will tend to fall, can that be changed to a tendency to rise by adding NO2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO 2 needed to reverse it. Round your answer to 2 significant digits. yes no 0.42 atm ☑ 5 0/5 ? مله Ararrow_forwardHomework 13 (Ch17) Question 4 of 4 (1 point) | Question Attempt: 2 of 2 ✓ 1 ✓ 2 = 3 4 Time Remaining: 4:25:54 Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: 2CH3OH (g)+302 (g) → 2CO2 (g) + 4H₂O (g) Round your answer to zero decimal places. ☐ kJ x10 ☐ Subm Check 2020 Hill LLC. All Rights Reserved. Terms of Use | Privacy Cearrow_forwardIdentifying the major species in weak acid or weak base equilibria Your answer is incorrect. • Row 2: Your answer is incorrect. • Row 3: Your answer is incorrect. • Row 6: Your answer is incorrect. 0/5 The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HF is a weak acid. acids: HF 0.1 mol of NaOH is added to 1.0 L of a 0.7M HF solution. bases: 0.13 mol of HCl is added to 1.0 L of a solution that is 1.0M in both HF and KF. Exponent other: F acids: HF bases: F other: K 1 0,0,... ? 000 18 Ararrow_forward
- Using reaction free energy to predict equilibrium composition Consider the following equilibrium: 2NOCI (g) 2NO (g) + Cl2 (g) AGº =41. kJ Now suppose a reaction vessel is filled with 4.50 atm of nitrosyl chloride (NOCI) and 6.38 atm of chlorine (C12) at 212. °C. Answer the following questions about this system: ? rise Under these conditions, will the pressure of NOCI tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding NO? In other words, if you said the pressure of NOCI will tend to rise, can that be changed to a tendency to fall by adding NO? Similarly, if you said the pressure of NOCI will tend to fall, can that be changed to a tendency to rise by adding NO? yes no If you said the tendency can be reversed in the second question, calculate the minimum pressure of NO needed to reverse it. Round your answer to 2 significant digits. 0.035 atm ✓ G 00. 18 Ararrow_forwardHighlight each glycosidic bond in the molecule below. Then answer the questions in the table under the drawing area. HO- HO- -0 OH OH HO NG HO- HO- OH OH OH OH NG OHarrow_forward€ + Suppose the molecule in the drawing area below were reacted with H₂ over a platinum catalyst. Edit the molecule to show what would happen to it. That is, turn it into the product of the reaction. Also, write the name of the product molecule under the drawing area. Name: ☐ H C=0 X H- OH HO- H HO- -H CH₂OH ×arrow_forward
- Draw the Haworth projection of the disaccharide made by joining D-glucose and D-mannose with a ẞ(1-4) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. Xarrow_forwardEpoxides can be opened in aqueous acid or aqueous base to produce diols (molecules with two OH groups). In this question, you'll explore the mechanism of epoxide opening in aqueous acid. 2nd attempt Be sure to show all four bonds at stereocenters using hash and wedge lines. 0 0 Draw curved arrows to show how the epoxide reacts with hydronium ion. 100 +1: 1st attempt Feedback Be sure to show all four bonds at stereocenters using hash and wedge lines. See Periodic Table See Hint H A 5 F F Hr See Periodic Table See Hintarrow_forward03 Question (1 point) For the reaction below, draw both of the major organic products. Be sure to consider stereochemistry. > 1. CH₂CH₂MgBr 2. H₂O 3rd attempt Draw all four bonds at chiral centers. Draw all stereoisomers formed. Draw the structures here. e 130 AN H See Periodic Table See Hint P C Brarrow_forward
- You may wish to address the following issues in your response if they are pertinent to the reaction(s) you propose to employ:1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Please make it in detail and draw it out too in what step what happens. Thank you for helping me!arrow_forward1) Chemoselectivity (why this functional group and not another?) 2) Regioselectivity (why here and not there?) 3) Stereoselectivity (why this stereoisomer?) 4) Changes in oxidation state. Everything in detail and draw out and write it.arrow_forwardCalculating the pH at equivalence of a titration 3/5 Izabella A chemist titrates 120.0 mL of a 0.7191M dimethylamine ((CH3)2NH) solution with 0.5501 M HBr solution at 25 °C. Calculate the pH at equivalence. The pk of dimethylamine is 3.27. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = ☐ ✓ 18 Ar Boarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





