
(a)
Interpretation:
It is to be predicted which of the two nucleophiles in the given pair will react faster with
Concept introduction:
The minimum amount of energy required by reacting molecules to reach the transition state is called the activation energy.
For nucleophilic substitution reactions, if the reactant is the same, the activation energy increases with increasing stability of the substitution product.
The reaction is faster when the energy barrier between the reactants and products is small. When energy difference between reactants and the transition state is smaller than the energy difference between products and transition state, the reactants are highly reactive and reaction is faster. Highly reactive reactant molecules serve as strong nucleophiles. The reactants are less reactive and the reaction slower, when the transition state is closer in energy to the products than the reactants.
Answer to Problem 9.4P
(i) Among the two nucleophiles, the nucleophile
(ii) Among the two nucleophiles, the nucleophile
Explanation of Solution
(i) The first given pair of nucleophiles is
The reactions of each of these nucleophiles with
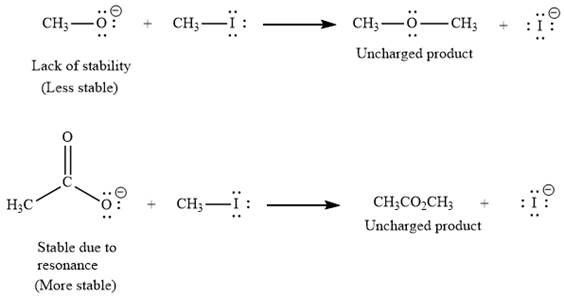
There is a significant difference in charge stability on the reactant side but not on the product side because the negative charge in
(ii) The second given pair of nucleophiles is
The reactions of each of these nucleophiles with
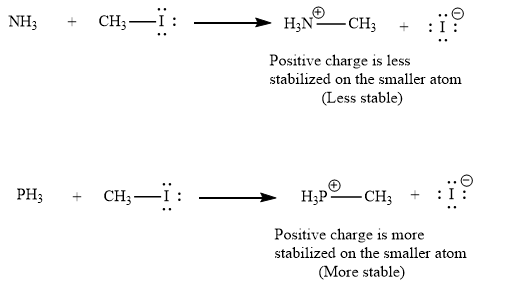
There is a significant difference in charge stability on the product side but not on the reactant side. Because a positive charge is more stable on phosphorous atom than on nitrogen atom, the product of the nucleophilic substitution involving
Therefore, the energy barrier involving
As nucleophilicity of the attacking species increases in
(b)
Interpretation:
It is to be determined which of the given pairs is the stronger nucleophile.
Concept introduction:
The minimum amount of energy required by reacting molecules to reach the transition state is called the activation energy. For nucleophilic substitution reactions, if the reactant is the same, the activation energy increases with increasing stability of the substitution product. When energy difference between reactants and the transition state is smaller than the energy difference between products and transition state, the reactants are highly reactive and reaction is faster. Highly reactive reactant molecules serves as strong nucleophiles.
Answer to Problem 9.4P
(i) Among the pair of nucleophiles,
(ii) Among the two nucleophiles,
Explanation of Solution
(i) The first given pair of nucleophiles is
In part (a), it is determined that the reaction of
(ii) The first given pair of nucleophiles is
In part (a), it is determined that the reaction of
Among the two given nucleophiles, the stronger nucleophile is the one that exhibits a faster reaction.
Want to see more full solutions like this?
Chapter 9 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- 4. Draw and label all possible isomers for [M(py)3(DMSO)2(CI)] (py = pyridine, DMSO dimethylsulfoxide).arrow_forwardThe emission data in cps displayed in Table 1 is reported to two decimal places by the chemist. However, the instrument output is shown in Table 2. Table 2. Iron emission from ICP-AES Sample Blank Standard Emission, cps 579.503252562 9308340.13122 Unknown Sample 343.232365741 Did the chemist make the correct choice in how they choose to display the data up in Table 1? Choose the best explanation from the choices below. No. Since the instrument calculates 12 digits for all values, they should all be kept and not truncated. Doing so would eliminate significant information. No. Since the instrument calculates 5 decimal places for the standard, all of the values should be limited to the same number. The other decimal places are not significant for the blank and unknown sample. Yes. The way Saman made the standards was limited by the 250-mL volumetric flask. This glassware can report values to 2 decimal places, and this establishes our number of significant figures. Yes. Instrumental data…arrow_forwardSteps and explanation pleasearrow_forward
- Try: Convert the given 3D perspective structure to Newman projection about C2 - C3 bond (C2 carbon in the front). Also, show Newman projection of other possible staggered conformers and circle the most stable conformation. Use the template shown. F H3C Br Harrow_forwardNonearrow_forward16. Consider the probability distribution p(x) = ax", 0 ≤ x ≤ 1 for a positive integer n. A. Derive an expression for the constant a, to normalize p(x). B. Compute the average (x) as a function of n. C. Compute σ2 = (x²) - (x)², the variance of x, as a function of n.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
