
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 9.41SP
The following functional-group interchange is a useful synthesis of

- a. What reagents were used in this chapter for this transformation? Give an example to illustrate this method.
- b. This functional-group interchange can also be accomplished using the following sequence.
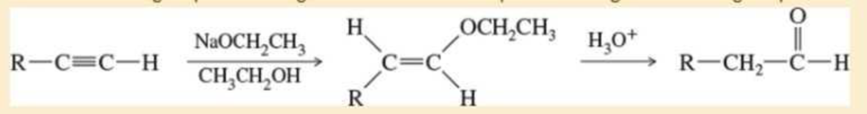
Propose mechanisms for these steps.
- c. Explain why a nucleophilic reagent such as ethoxide adds to an
alkyne more easily than it adds to analkene .
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
4. Provide a synthetic route to the following molecule using benzene and cyclohexane.
Reagents cannot contain more than one carbon. Provide a mechanism for the last step
of your synthetic route.
A. Draw the products of the reaction shown below. Use wedge and dish bonds to indicate the dicarbonyl starting material of the reaction shown below. Ignore inorganic
stereochemistry. Ignore inorganic byproducts.
byproducts.
Draw the products resulting from addition of a Grignard reagent to an aldehyde. D. Draw the products resulting from addition of a Grignard reagent to an aldehydom
Use a dash or wedge bond to indicate the stereochemistry of substituents on
asymmetric centers, where applicablelgnore any inorganic byproducts.
Use a dash or wedge bond to indicate the stereochemistry of substituents on
asymmetric centers, where applicablelgnore any inorganic byproducts.
1.00
2. NHSO HO
2.HO
Select to Draw
1) PhMgCl (CHMgC
CH
2) HCI/H:O
++
1) vinylmagnesium chloride (CH=CHC
2HC/H.O
Select to Edit
OH
Select to Edit
Select to Ede
Propose reagents and conditions for the following multistep syntheses. You may use any reagents
necessary. Be sure to draw all isolable intermediates.
Br.
H.
Chapter 9 Solutions
Organic Chemistry (9th Edition)
Ch. 9.1 - a. Count the elements of unsaturation in...Ch. 9.2 - Prob. 9.2PCh. 9.4B - What reaction would acetylene likely undergo if it...Ch. 9.6 - Prob. 9.4PCh. 9.6 - Predict the products of the following acid-base...Ch. 9.7A - Solved Problem9-1 showed the synthesis of...Ch. 9.7A - Show how you might synthesize the following...Ch. 9.7B - Prob. 9.8PCh. 9.7B - Show how you would synthesize...Ch. 9.8 - When 2,2-dibromo-1-phenylpropane is heated...
Ch. 9.8 - When 2,2-dibromo-1-phenylpropane is heated...Ch. 9.9C - Show how you would convert a. oct-3-yne to...Ch. 9.9C - The fragrance of (Z)-1-phenylhex-2-en-1-ol...Ch. 9.9D - In the addition of just 1 mole of bromine to 1...Ch. 9.9E - Propose a mechanism for the entire reaction of...Ch. 9.9E - Predict the major product(s) of the following...Ch. 9.9E - Propose a mechanism for the reaction of pent-1-yne...Ch. 9.9E - Show how hex-1-yne might be converted to a....Ch. 9.9F - When pent-2-yne reacts with mercuric sulfate in...Ch. 9.9F - Prob. 9.20PCh. 9.9F - Prob. 9.21PCh. 9.9F - Prob. 9.22PCh. 9.10A - Predict the product(s) you would expect from...Ch. 9.10B - Prob. 9.24PCh. 9.10B - Prob. 9.25PCh. 9 - Prob. 9.26SPCh. 9 - Give common names for the following compounds. a....Ch. 9 - Prob. 9.28SPCh. 9 - Prob. 9.29SPCh. 9 - Using cyclooctyne as your starting material, show...Ch. 9 - Prob. 9.31SPCh. 9 - Prob. 9.32SPCh. 9 - Predict the products of reaction of pent-1-yne...Ch. 9 - Show how you would accomplish the following...Ch. 9 - Show how you would synthesize the following...Ch. 9 - Predict the products formed when CH3CH2C C : Na+...Ch. 9 - Prob. 9.37SPCh. 9 - Prob. 9.38SPCh. 9 - When compound Z is treated with ozone, followed by...Ch. 9 - Show how you would convert the following starting...Ch. 9 - The following functional-group interchange is a...Ch. 9 - Using any necessary inorganic reagents, show how...Ch. 9 - Prob. 9.43SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Why is it necessary to be in a pressurized cabin when flying at 30,000 feet?
Anatomy & Physiology (6th Edition)
Give the IUPAC name for each compound.
Organic Chemistry
Define histology.
Fundamentals of Anatomy & Physiology (11th Edition)
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- See image belowarrow_forward1. Show all steps in the synthesis of 4-methylaniline from toluene. Clearly show all reagents, reactants and products for each step. Toluene -------> --------> 4-methylanilinearrow_forwardSynthesize the following compounds from the given starting material. You can add on any alkyl/ alkyl halide under or equal to 4 carbons or any necessary inorganic reagent needed (this includes triphenyl phosphine (Ph3P). Please draw all intermediates and reagents necessary to get to the product. b. sobarrow_forward
- 6. Multi Step Synthesis. Propose a synthetic route from the reactant to the product using any reagents you need. Nalt LINH4arrow_forward6. Show how to synthesize the target molecule from the given material as the only source of carbon. Show the reagents needed for each step and the product of each step. OH .OHarrow_forward6. Show how you would carry out the following transformations as shown. More than one step is required in each step. Show all steps clearly with reactants, reagents and products. a. b. OH ? ? OH OHarrow_forward
- Write equations for the preparation of the following alkenes via the Wittig reaction. Any carbonyl and ylide will workarrow_forwardButanone undergoes a nucleophilic addition with a Grignard reagent made from 1-bromopropane and magnesium metal in THF solution. The alkoxide formed from the nucleophilic addition is then conveted into the final product by the careful addition of dilute acid. Complete the mechanism by following the instructions provided for each step. Step 1. Nucleophilic Addition in THFarrow_forward13arrow_forward
- Draw the major product of this reaction. Ignore inorganic byproducts. 1. Hg(OAc)2, H₂O 2. NaBH4, NaOH Drawing atarrow_forwardComplete the following reaction schemes. Some may take more than one step and some may have several routes to the product.arrow_forwardIs the product of this reaction more or less soluble than the initial molecule? Why? 1. RCO;H 2. H*, H,O Less water-soluble More water-soluble Water-solubility improved due to a negative charge on the product. Water-solubility improved due to a positive charge on the product. The product now has sufficient H-bonding functional groups to support water-solubility. An acetal has been formed which would decrease the water-solubility Another reasonarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License