
Concept explainers
(a)
Interpretation:
The condensed electron configurations and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(a)
Answer to Problem 9.21P
The condensed electronic configuration of
The condensed electronic configuration of
The Lewis orbital diagram is as follows:
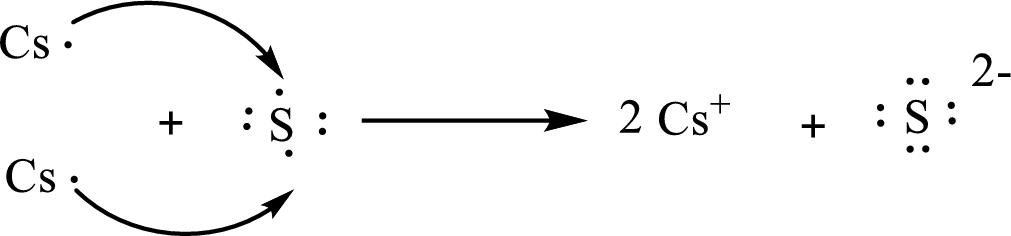
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of a cesium atom
The condensed electronic configuration of the sulfur atom
Two cesium atoms lose one electron respectively to form
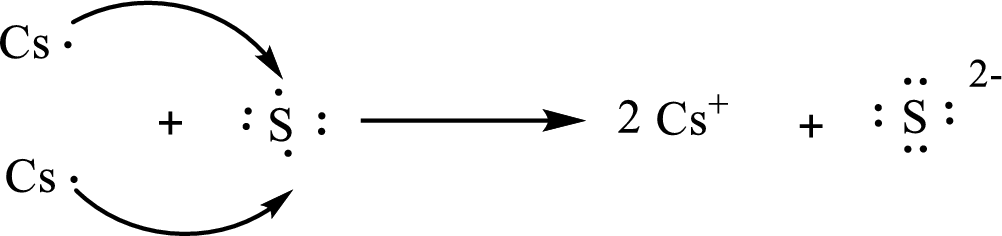
The condensed electronic configuration of
The condensed electronic configuration of
Two cesium atoms lose one electron respectively to form
The Lewis orbital diagram is as follows:
(b)
Interpretation:
The condensed electron configurations, partial orbital diagrams, and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is a pictorial representation of the electrons present in an orbital. Each orbital can occupy only two electrons of opposite spin.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(b)
Answer to Problem 9.21P
The condensed electronic configuration of
The condensed electronic configuration of
The Lewis orbital diagram is as follows:
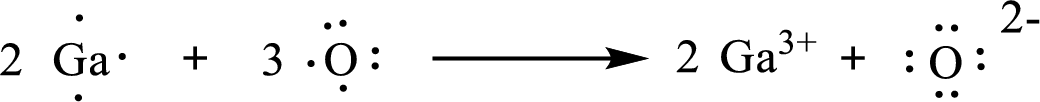
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of gallium atom
The condensed electronic configuration of oxygen atom
Two gallium atoms lose three electrons respectively to form
The condensed electronic configuration of
The condensed electronic configuration of
Two gallium atoms lose three electrons respectively to form
The Lewis orbital diagram is as follows:

(c)
Interpretation:
The condensed electron configurations, partial orbital diagrams, and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is a pictorial representation of the electrons present in an orbital. Each orbital can occupy only two electrons of opposite spin.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(c)
Answer to Problem 9.21P
The condensed electronic configuration of
The condensed electronic configuration of
The Lewis orbital diagram is as follows:
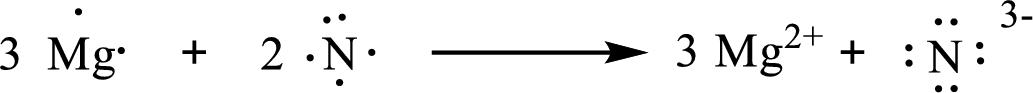
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of magnesium atom
The condensed electronic configuration of nitrogen atom
Three magnesium atoms lose two electrons respectively to form
The condensed electronic configuration of
The condensed electronic configuration of
Three magnesium atoms lose two electrons respectively to form
The Lewis orbital diagram is as follows:
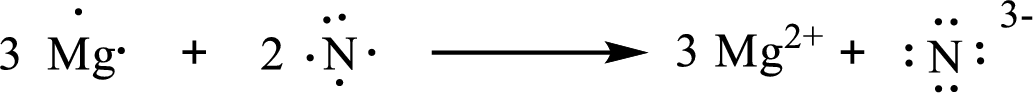
(d)
Interpretation:
The condensed electron configurations, partial orbital diagrams, and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is a pictorial representation of the electrons present in an orbital. Each orbital can occupy only two electrons of opposite spin.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(d)
Answer to Problem 9.21P
The condensed electronic configuration of
The condensed electronic configuration of
The Lewis orbital diagram is as follows:
The formula of the compound formed is
Explanation of Solution
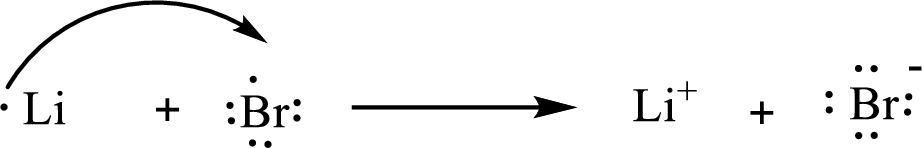
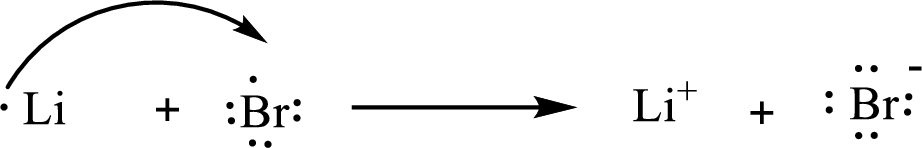
The condensed electronic configuration of a lithium atom
The condensed electronic configuration of the bromine atom
Lithium atom loses one electron to form
The condensed electronic configuration of
The condensed electronic configuration of
Lithium atom loses one electron to form
The Lewis orbital diagram is as follows:
Want to see more full solutions like this?
Chapter 9 Solutions
Chemistry: The Molecular Nature of Matter and Change - Standalone book
- Ideally nonpolarizable electrodes can1. participate as reducers in reactions.2. be formed only with hydrogen.3. participate as oxidizers in reactions.4. form open and closed electrochemical systems.arrow_forwardIndicate the options for an electrified interface:1. Temperature has no influence on it.2. Not all theories that describe it include a well-defined electrical double layer.3. Under favorable conditions, its differential capacitance can be determined with the help of experimental measurements.4. A component with high electronic conductivity is involved in its formation.arrow_forwardTo describe the structure of the interface, there are theories or models that can be distinguished by:1. calculation of the charge density.2. distribution of ions in the solution.3. experimentally measured potential difference.4. external Helmoltz plane.arrow_forward
- Indicate the correct options when referring to Luther's equation:1. It is not always easy to compare its results with experimental results.2. It depends on the number of electrons exchanged in the species involved.3. Its foundation is thermodynamic.4. The values calculated with it do not depend on temperature.arrow_forwardIndicate which of the unit options correspond to a measurement of current density.1. A s m-22. mC s-1 m-23. Ω m-24. V J-1 m-2arrow_forwardIndicate the options that are true when referring to electrode membranes:1. The Donnan potential, in general, does not always intervene in membranes.2. There are several ways to classify the same membrane.3. Any membrane can be used to determine the pH of a solution.4. Only one solution and one membrane are needed to determine the pH of that solution.arrow_forward
- Calculate the maximum volume of carbon dioxide gasarrow_forwardIn galvanic cells, their potential1. can be measured with a potentiometer2. does not depend on the equilibrium constant of the reaction occurring within them3. is only calculated from the normal potentials of the electrodes they comprise4. can sometimes be considered a variation in a potential differencearrow_forwardIf some molecules in an excited state collide with other molecules in a ground state, this process1. can occur in solution and in the gas phase.2. can be treated as a bimolecular process.3. always results in collisional deactivation.4. does not compete with any other process.arrow_forward
- Radiation of frequency v is incident on molecules in their ground state. The expected outcome is that1. the molecules do not change their state.2. the molecules transition to an excited state.3. the molecules undergo a secondary process.4. collisional deactivation occurs.arrow_forwardPredict the major product of the following reaction and then draw a curved arrow mechanism for its formation. Part: 0/2 Part 1 of 2 H₂SO heat : OH 90 Draw the structure of the major product. Click and drag to start drawing a structure. 3arrow_forwardDraw a curved arrow mechanism for the reaction, adding steps as necessary. Be sure to include all electrons that are necessary to the mechanism and all nonzero formal charges. C Ö-H H + -S-OH .0. Add/Remove step X टे Click and drag to start drawing a structure.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





