
Concept explainers
(a)
Interpretation:
Taking a basis of 1 mol of feed gas, the moles of each component of the feed product mixture and extent of reaction should be calculated.
Concept Introduction
The flow chart for the given situation would be helpful to solve the problem.
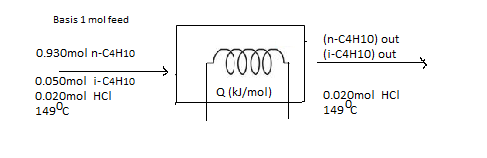
(b)
Interpretation:
First, the calculation of the standard heat of the isomerization reaction (kJ) should be found. Then, taking the feed and product species at 25? as references, an inlet-outlet enthalpy table should be prepared and component amounts (mol) and specific enthalpies (kJ/mol) should be calculated and filled in.
Concept introduction:
The standard heat of formation will be helpful to solve the problem as:
(c)
Interpretation:
The amount of heat transferred should be calculated in kJ and the required heat transfer for a reactor feed of 325 kJ/h should be determined in kW.
Concept introduction:
The heat transferred is calculated using formula:
(d)
Interpretation:
The calculated results should be used to estimate the heat of the isomerization reaction at 149? and the assumptions built into the estimation should be listed.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
ELEM.PRIN.OF CHEMICAL...ABRIDGED (LL)
- Q2: Draw the layout of a basic asynchronous 32k * 8 SRAM.arrow_forwardA distillation column with 100 kmol/h feed of 50% A and 50% B produces a distillate product with xD = 0.95 and a bottom stream with xbot = 0.04 of the more volatile species A. CMO is valid and the equilibrium data is given by y = 2.4x/1 + 1.4x a) If the feed is saturated liquid, determine the minimum reflux ratio b) If the feed is saturated vapor, determine the minimum reflux ratioarrow_forwardA distillation column with 100 kmol/h feed of 60% A and 40% B produces a distillate product with xD = 0.98 and a bottom stream with xbot = 0.02 of the more volatile species A. CMO is valid and the equilibrium data is given by y = 2.2x/1+1.2x a) If the reflux ratio R is 2, determine (numerically) the composition (y) of the vapor stream entering the top equilibrium plate.__________b) If R = 2 and q = 0.6, determine the liquid flow rate in the stripping section of the column__________c) If q = 0, the minimum reflux ratio isarrow_forward
- Natural gas having a specific gravity relative to air of 0.60 and a viscosity of 0.011 cP is flowing through a 6-in. Schedule 40 pipe in which is installed a standard sharp-edged orifice equipped with flange taps. The gas is at 100°F and 20lb/in? abs at the upstream tap. The manometer reading is 46.3 in. of water at 60°F. The ratio of specific heats for natural gas is 1.30. The diameter of the orifice is 2.00 in. Calculate the rate of flow of gas through the line in cubic feet.arrow_forwardصورة من s94850121arrow_forward11:01 ☑ canvas.ucsd.edu 口 : ... Page 1 > of 2 Q - ZOOM + 4. Consider the two separate sets of measured data for a silt-loam soil measured by Mualem (1976): (1) suction versus water content, and (2) suction versus relative permeability of unsaturated soil, k/ks. Assume that 0s 0.396, 0res = 0.131, and Ks=5.74×10-7 m/s. a. Using the method of least squares in Excel, compute the best-fit values for αNG (kPa¹) and nvg for the van Genuchten (1980) relationship for data set # 1 (assume m = 1-1/nvG). See the example spreadsheet in the homework folder under the files section of Canvas for help in performing this calculation. b. Repeat part (a) and estimate the λ and ac parameters for the Brooks and Corey (1964) SWRC for data set #1. Note that you may need to include an "if" statement at the air entry suction. c. Plot the data for the SWRC versus the fitted van Genuchten (1980) and Brooks and Corey (1964) curves. Which relationship matches the capillary pressure data better (BC or VG)? Explain…arrow_forward
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





