
EP BASIC CHEMISTRY-STANDALONE ACCESS
6th Edition
ISBN: 9780134999890
Author: Timberlake
Publisher: PEARSON CO
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 53UTC
The chapter sections lo review are shown in parentheses at the end of each problem
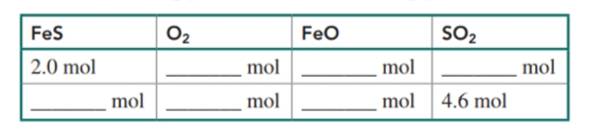
Use the balanced chemical equation to complete the table: (9.1,9.2)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
reciprocal lattices rotates along with the real space lattices of the crystal. true or false?
Deducing the reactants of a Diels-Alder reaction
vn the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one
step, by moderately heating the reactants?
?
Δ
O
If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any
arrangement you like.
• If your answer is no, check the box under the drawing area instead.
Click and drag to start drawing a structure.
Product can't be made in one step.
Explanation
Check
Predict the major products of the following organic reaction:
Δ
?
Some important notes:
• Draw the major product, or products, of the reaction in the drawing area below.
• If there aren't any products, because no reaction will take place, check the box below the drawing area instead.
• Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are
enantiomers.
Explanation
Check
Click and drag to start drawing a structure.
L
Chapter 9 Solutions
EP BASIC CHEMISTRY-STANDALONE ACCESS
Ch. 9.1 - Calculate the total mass of the reactants and the...Ch. 9.1 - Calculate the total mass of the reactants and the...Ch. 9.2 - Calculate the total mass of the reactants and the...Ch. 9.2 - Write all of the mole-mole factors for each of the...Ch. 9.2 - For the chemical equations in problem 9.3, write...Ch. 9.2 - For the chemical equations in problem 9.4, write...Ch. 9.2 - The chemical reaction of hydrogen with oxygen...Ch. 9.2 - Ammonia is produced by the chemical reaction of...Ch. 9.2 - Carbon disulfide and carbon monoxide are produced...Ch. 9.2 - In the acetylene torch, acetylene gas burns in...
Ch. 9.3 - Sodium reacts with oxygen to produce sodium oxide....Ch. 9.3 - Nitrogen reacts with hydrogen to produce ammonia....Ch. 9.3 - Ammonia and oxygen react to form nitrogen and...Ch. 9.3 - Iron(III) oxide reacts with carbon to give iron...Ch. 9.3 - Prob. 15PPCh. 9.3 - Calcium cyanamide, CaCN2 , reacts with water to...Ch. 9.3 - Prob. 17PPCh. 9.3 - Prob. 18PPCh. 9.4 - A taxi company has 10 taxis. a. On a certain day,...Ch. 9.4 - Prob. 20PPCh. 9.4 - Prob. 21PPCh. 9.4 - Iron and oxygen react to form iron(III) oxide....Ch. 9.4 - For each of the following reactions, 20.0 g of...Ch. 9.4 - For each of the following reactions, 20.0 g of...Ch. 9.4 - For each of the following reactions, calculate the...Ch. 9.4 - For each of the following reactions, calculate the...Ch. 9.5 - Carbon disulfide is produced by the reaction of...Ch. 9.5 - Iron (III) oxide reacts with carbon monoxide to...Ch. 9.5 - Aluminum reacts with oxygen to produce aluminum...Ch. 9.5 - Propane ( C3H8 ) bums in oxygen to produce carbon...Ch. 9.5 - Prob. 31PPCh. 9.5 - When 56.6 g of calcium is reacted with nitrogen...Ch. 9.6 - In an exothermic reaction, is the energy of the...Ch. 9.6 - In an endothermic reaction, is the energy of the...Ch. 9.6 - Prob. 35PPCh. 9.6 - Prob. 36PPCh. 9.6 - Classify each of the following as exothermic or...Ch. 9.6 - Classify each of the following as exothermic or...Ch. 9.6 - Prob. 39PPCh. 9.6 - a. How many kilojoules are released when 75.0 g of...Ch. 9.6 - Prob. 41PPCh. 9.6 - Calculate the energy change for the reaction...Ch. 9.6 - Prob. 43PPCh. 9.6 - Prob. 44PPCh. 9.6 - In one step in the synthesis of the insecticide...Ch. 9.6 - Another widely used insecticide is carbofuran...Ch. 9 - The chapter sections lo review are shown in...Ch. 9 - The chapter sections lo review are shown in...Ch. 9 - The chapter sections lo review are shown in...Ch. 9 - The chapter sections lo review are shown in...Ch. 9 - The chapter sections lo review are shown in...Ch. 9 - The chapter sections lo review are shown in...Ch. 9 - The chapter sections lo review are shown in...Ch. 9 - The chapter sections lo review are shown in...Ch. 9 - Prob. 55APPCh. 9 - Prob. 56APPCh. 9 - Prob. 57APPCh. 9 - Prob. 58APPCh. 9 - Prob. 59APPCh. 9 - Prob. 60APPCh. 9 - Pentane gas ( C5H12 ) reacts with oxygen to...Ch. 9 - Prob. 62APPCh. 9 - Prob. 63APPCh. 9 - Prob. 64APPCh. 9 - Prob. 65APPCh. 9 - Prob. 66APPCh. 9 - Prob. 67APPCh. 9 - Prob. 68APPCh. 9 - Prob. 69APPCh. 9 - The equation for the reaction of iron and oxygen...Ch. 9 - Prob. 71APPCh. 9 - Prob. 72APPCh. 9 - Prob. 73CPCh. 9 - Prob. 74CPCh. 9 - Prob. 75CPCh. 9 - The following problems are related to the topics...Ch. 9 - Prob. 77CPCh. 9 - Prob. 78CPCh. 9 - Prob. 79CPCh. 9 - Prob. 80CPCh. 9 - The following problems are related to the topics...Ch. 9 - Prob. 82CPCh. 9 - Prob. 83CPCh. 9 - The following problems are related to the topics...Ch. 9 - Prob. 85CPCh. 9 - Prob. 86CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- > Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? ? Δ • If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. If your answer is no, check the box under the drawing area instead. Explanation Check Click and drag to start drawing a structure. Х © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accesarrow_forwardPredict the major products of the following organic reaction: O O + A ? Some important notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. Explanation Check Click and drag to start drawing a structure. eserved. Terms of Use | Privacy Center >arrow_forward(EXM 2, PRBLM 3) Here is this problem, can you explain it to me and show how its done. Thank you I need to see the work for like prbl solving.arrow_forward
- can someone draw out the reaction mechanism for this reaction showing all bonds, intermediates and side products Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided belowarrow_forwardWhat would be the best choices for the missing reagents 1 and 3 in this synthesis? 1. PPh3 3 2. n-BuLi • Draw the missing reagents in the drawing area below. You can draw them in any arrangement you like. • Do not draw the missing reagent 2. If you draw 1 correctly, we'll know what it is. • Note: if one of your reagents needs to contain a halogen, use bromine. Click and drag to start drawing a structure.arrow_forwardIdentify the missing organic reactants in the following reaction: X + Y H+ two steps Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H2O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. Х :arrow_forward
- I am struggling with the IUPAC (sys H Reply ☑Mark as Unreadarrow_forwardDon't used hand raiting and don't used Ai solution and correct answerarrow_forwardH R Part: 1/2 :CI: is a/an electrophile Part 2 of 2 Draw the skeletal structure of the product(s) for the Lewis acid-base reaction. Include lone pairs and formal charges (if applicable) on the structures. 4-7: H ö- H Skip Part Check X :C1: $ % L Fi Click and drag to start drawing a structure. MacBook Pro & ㅁ x G 0: P Add or increase positive formal cha Save For Later Submit ©2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY