
Modified Mastering Physics with Pearson eText -- Access Card -- for Physics (18-Weeks)
5th Edition
ISBN: 9780136781356
Author: Walker, JAMES
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 9, Problem 41PCE
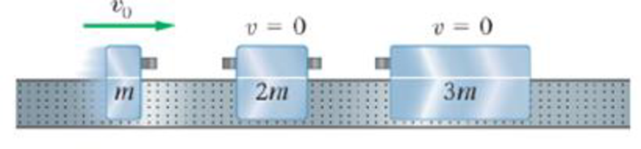
An air-track cart with mass m =0.25 kg and speed v0 = 1.2 m/s approaches two other carts that are at rest and have masses 2m and 3m as indicated in Figure 9-29. The carts have bumpers that make all the collisions elastic. Find the final speed of each of the carts, assuming the air track extends indefinitely in either direction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A bobsled starts at the top of a track as human runners sprint from rest and then jump into the sled. Assume they reach 40 km/h from rest after covering a distance of 50 m over flat ice. a. How much work do they do on themselves and the sled which they are pushing given the fact that there are two men of combined mass 185 kg and the sled with a mass of 200 kg? (If you haven't seen bobsledding, watch youtube to understand better what's going on.) b. After this start, the team races down the track and descends vertically by 200 m. At the finish line the sled crosses with a speed of 55 m/s. How much energy was lost to drag and friction along the way down after the men were in the sled?
For what type of force is it not possible to define a potential energy expression?
10. Imagine you have a system in which you have 54 grams of ice. You can melt this
ice and then vaporize it all at 0 C. The melting and vaporization are done reversibly
into a balloon held at a pressure of 0.250 bar. Here are some facts about water you
may wish to know. The density of liquid water at 0 C is 1 g/cm³. The density of ice at 0
C is 0.917 g/cm³. The enthalpy of vaporization of liquid water is 2.496 kJ/gram and the
enthalpy of fusion of solid water is 333.55 J/gram.
Chapter 9 Solutions
Modified Mastering Physics with Pearson eText -- Access Card -- for Physics (18-Weeks)
Ch. 9.1 - Enhance Your Understanding (Answers given at the...Ch. 9.2 - Enhance Your Understanding (Answers given at the...Ch. 9.3 - Enhance Your Understanding (Answers given at the...Ch. 9.4 - Enhance Your Understanding (Answers given at the...Ch. 9.5 - Prob. 5EYUCh. 9.6 - Enhance Your Understanding (Answers given at the...Ch. 9.7 - Enhance Your Understanding (Answers given at the...Ch. 9.8 - Prob. 8EYUCh. 9 - If you drop your Keys, their momentum increases as...Ch. 9 - By what factor does an objects kinetic energy...
Ch. 9 - A system of particles is known to have zero...Ch. 9 - A system of particles is known to have zero...Ch. 9 - On a calm day you connect an electric fan to a...Ch. 9 - Crash statistics show that it is safer to be...Ch. 9 - (a) As you approach a stoplight, you apply the...Ch. 9 - An object at rest on a frictionless surface is...Ch. 9 - (a) Can two objects on a horizontal frictionless...Ch. 9 - Two cars collide at an intersection. If the cars...Ch. 9 - At the instant a bullet is fired from a gun, the...Ch. 9 - Prob. 12CQCh. 9 - In the classic movie The Spirit of St. Louis,...Ch. 9 - A tall, slender drinking glass with a thin base is...Ch. 9 - Prob. 15CQCh. 9 - Prob. 16CQCh. 9 - What is the mass of a mallard duck whose speed is...Ch. 9 - (a) What is the magnitude of the momentum of a...Ch. 9 - A 54 kg person walks due north with a speed of 1.2...Ch. 9 - A 26.2-kg dog is running northward at 2.70 m/s,...Ch. 9 - Predict/Calculate Two air-track carts move toward...Ch. 9 - A 0.145-kg baseball is dropped from rest. If the...Ch. 9 - A 285-g ball falls vertically downward, hitting...Ch. 9 - Object 1 has a mass m1 and a velocity...Ch. 9 - Your car rolls slowly in a parking lot and bangs...Ch. 9 - Predict/Explain A net force of 200 N acts on a...Ch. 9 - Predict/Explain Referring to the previous...Ch. 9 - Predict/Explain Two identical cars, each traveling...Ch. 9 - Force A has a magnitude F and acts for the time t...Ch. 9 - Find the magnitude of the impulse delivered to a...Ch. 9 - A 0.45-kg croquet ball is initially at rest on the...Ch. 9 - When spiking a volleyball, a player changes the...Ch. 9 - Force Platform A force platform measures the...Ch. 9 - Air Bag Safety If a driver makes contact with a...Ch. 9 - To make a bounce pass, a player throws a 0.60-kg...Ch. 9 - BIO Concussion Impulse One study suggests that a...Ch. 9 - Predict/Calculate A 0.14-kg baseball moves toward...Ch. 9 - A player bounces a 0.43-kg soccer ball off her...Ch. 9 - Two ice skaters stand at rest in the center of an...Ch. 9 - A 0.042-kg pet lab mouse sits on a 0.35-kg...Ch. 9 - An object initially at rest breaks into two pieces...Ch. 9 - A 92-kg astronaut and a 1200-kg satellite are at...Ch. 9 - The recoil of a shotgun can be significant....Ch. 9 - A plate drops onto a smooth floor and shatters...Ch. 9 - Suppose the car in Example 9-13 has an initial...Ch. 9 - Two 78.5-kg hockey players skating at 4.47 m/s...Ch. 9 - An air-track cart with mass m1 = 0.32 kg and...Ch. 9 - Predict/Calculate A bullet with a mass of 4.0 g...Ch. 9 - BIO Concussion Recoil The human head can be...Ch. 9 - Two objects moving with a speed v travel in...Ch. 9 - In the apple-orange collision in Example 9-16,...Ch. 9 - A732-kg car stopped at an intersection is...Ch. 9 - The collision between a hammer and a nail can be...Ch. 9 - Predict/Calculate A charging bull elephant with a...Ch. 9 - Prob. 39PCECh. 9 - The three air carts shown in Figure 9-28 have...Ch. 9 - An air-track cart with mass m =0.25 kg and speed...Ch. 9 - Predict/Explain A stalactite in a cave has drops...Ch. 9 - Prob. 43PCECh. 9 - Find the x coordinate of the center of mass of the...Ch. 9 - Prob. 45PCECh. 9 - A pencil standing upright on its eraser end falls...Ch. 9 - Prob. 47PCECh. 9 - The location of the center of mass of the...Ch. 9 - The Center of Mass of Sulfur Dioxide Sulfur...Ch. 9 - Prob. 50PCECh. 9 - A 0 726-kg rope 2 00 meters long lies on a floor...Ch. 9 - Prob. 52PCECh. 9 - Prob. 53PCECh. 9 - Helicopter Thrust During a rescue operation, a...Ch. 9 - Rocks for a Rocket Engine A child sits in a wagon...Ch. 9 - A 57.8-kg person holding two 0.880-kg bricks...Ch. 9 - A fire hose can expel water at a rate of 9.5 kg/s...Ch. 9 - A 0 540-kg bucket rests on a scale Into this...Ch. 9 - Predict/Calculate Holding a long rope by its upper...Ch. 9 - CE Object A has a mass m, object B has a mass 2m,...Ch. 9 - CE Object A has a mass m, object B has a mass 4m,...Ch. 9 - CE A juggler performs a series of tricks with...Ch. 9 - A golfer attempts a birdie putt, sending the 0...Ch. 9 - Predict/Calculate Two trucks drive directly away...Ch. 9 - Prob. 65GPCh. 9 - A 1 35-kg block of wood sits at the edge of a...Ch. 9 - In a stunt, three people jump off a platform and...Ch. 9 - Predict/Calculate The carton of eggs shown in...Ch. 9 - The Force of a Storm During a severe storm in Palm...Ch. 9 - An experiment is performed in which two air carts...Ch. 9 - Figure 9-40 shows position-versus-time data from...Ch. 9 - To balance a 35.5-kg automobile tire and wheel, a...Ch. 9 - A hoop of mass M and radius R rests on a smooth,...Ch. 9 - Predict/Calculate A 63-kg canoeist stands in the...Ch. 9 - Prob. 75GPCh. 9 - A young hockey player stands at rest on the ice...Ch. 9 - Prob. 77GPCh. 9 - A 0.454-kg block is attached to a horizontal...Ch. 9 - BIO Escaping Octopus The giant Pacific octopus...Ch. 9 - Prob. 80GPCh. 9 - The three air carts shown in Figure 9-44 have...Ch. 9 - Unlimited Overhang Four identical textbooks, each...Ch. 9 - Consider a one-dimensional. head-on elastic...Ch. 9 - Two air carts of mass m1 = 0.84 kg and m2 = 0.42...Ch. 9 - Golden Earrings and the Golden Ratio A popular...Ch. 9 - Amplified Rebound Height Two small rubber balls...Ch. 9 - Predict/Calculate Weighing a Block on an Incline A...Ch. 9 - Predict/Calculate A uniform rope of length L and...Ch. 9 - Prob. 89PPCh. 9 - Prob. 90PPCh. 9 - Prob. 91PPCh. 9 - Prob. 92PPCh. 9 - Referring to Example 9-12 Suppose a bullet of mass...Ch. 9 - Referring to Example 9-12 A bullet with a mass m =...Ch. 9 - Referring to Example 9-19 Suppose that cart 1 has...Ch. 9 - Referring to Example 9-19 Suppose the two carts...
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. What are the primary functions of the skeletal system?
Human Anatomy & Physiology (2nd Edition)
8. A human maintaining a vegan diet (containing no animal products) would be a:
a. producer
b. primary consume...
Human Biology: Concepts and Current Issues (8th Edition)
APPLY 1.2 Express the following quantities in scientific notation
using fundamental SI units of mass and lengt...
Chemistry (7th Edition)
1. ___ Mitosis 2. ___ Meiosis 3. __ Homologous chromosomes 4. __ Crossing over 5. __ Cytokinesis A. Cytoplasmic...
Microbiology with Diseases by Body System (5th Edition)
Consider the reaction 4HCI(g)+O2(g)2H2O(g)+2Cl2(g) Each molecular diagram represents an initial mixture of the ...
Introductory Chemistry (6th Edition)
How do you think a cell performing cellular respiration rids itself of the resulting CO2?
Campbell Biology (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Consider 1 mole of supercooled water at -10°C. Calculate the entropy change of the water when the supercooled water freezes at -10°C and 1 atm. Useful data: Cp (ice) = 38 J mol-1 K-1 Cp (water) 75J mol −1 K -1 Afus H (0°C) 6026 J mol −1 Assume Cp (ice) and Cp (water) to be independent of temperature.arrow_forwardThe molar enthalpy of vaporization of benzene at its normal boiling point (80.09°C) is 30.72 kJ/mol. Assuming that AvapH and AvapS stay constant at their values at 80.09°C, calculate the value of AvapG at 75.0°C, 80.09°C, and 85.0°C. Hint: Remember that the liquid and vapor phases will be in equilibrium at the normal boiling point.arrow_forward3. The entropy of an ideal gas is S = Nkg In V. Entropy is a state function rather than a path function, and in this problem, you will show an example of the entropy change for an ideal gas being the same when you go between the same two states by two different pathways. A. Express ASV = S2 (V2) - S₁(V1), the change in entropy upon changing the volume from V₁to V2, at fixed particle number N and energy, U. B. Express ASN = S₂(N₂) - S₁ (N₁), the change in entropy upon changing the particle number from N₁ to N2, at fixed volume V and energy U. C. Write an expression for the entropy change, AS, for a two-step process (V₁, N₁) → (V2, N₁) → (V2, N₂) in which the volume changes first at fixed particle number, then the particle number changes at fixed volume. Again, assume energy is constant.arrow_forward
- Please don't use Chatgpt will upvote and give handwritten solutionarrow_forward6. We used the constant volume heat capacity, Cv, when we talked about thermodynamic cycles. It acts as a proportionality constant between energy and temperature: dU = C₁dT. You can also define a heat capacity for constant pressure processes, Cp. You can think of enthalpy playing a similar role to energy, but for constant pressure processes δαρ C = (37) - Sup Ср ат P = ат Starting from the definition of enthalpy, H = U + PV, find the relationship between Cy and Cp for an ideal gas.arrow_forwardPure membranes of dipalmitoyl lecithin phospholipids are models of biological membranes. They melt = 41°C. Reversible melting experiments indicate that at Tm AHm=37.7 kJ mol-1. Calculate: A. The entropy of melting, ASm- B. The Gibbs free energy of melting, AGm- C. Does the membrane become more or less ordered upon melting? D. There are 32 rotatable CH2 CH2 bonds in each molecule that can rotate more freely if the membrane melts. What is the increase in multiplicity on melting a mole of bonds?arrow_forward
- 5. Heat capacity often has a temperature dependence for real molecules, particularly if you go over a large temperature range. The heat capacity for liquid n-butane can be fit to the equation Cp(T) = a + bT where a = 100 J K₁₁ mol¹ and b = 0.1067 J K² mol¹ from its freezing point (T = 140 K) to its boiling point (T₁ = 270 K). A. Compute AH for heating butane from 170 K to 270 K. B. Compute AS for the same temperature range.arrow_forward4. How much energy must be transferred as heat to cause the quasi-static isothermal expansion of one mole of an ideal gas at 300 K from PA = 1 bar to PB = 0.5 bar? A. What is VA? B. What is VB? C. What is AU for the process? D. What is AH for the process? E. What is AS for the process?arrow_forward1. The diagram shows the tube used in the Thomson experiment. a. State the KE of the electrons. b. Draw the path of the electron beam in the gravitational field of the earth. C. If the electric field directed upwards, deduce the direction of the magnetic field so it would be possible to balance the forces. electron gun 1KVarrow_forward
- as a hiker in glacier national park, you need to keep the bears from getting at your food supply. You find a campground that is near an outcropping of ice. Part of the outcropping forms a feta=51.5* slopeup that leads to a verticle cliff. You decide that this is an idea place to hang your food supply out of bear reach. You put all of your food into a burlap sack, tie a rope to the sack, and then tie a bag full of rocks to the other end of the rope to act as an anchor. You currently have 18.5 kg of food left for the rest of your trip, so you put 18.5 kg of rocks in the anchor bag to balance it out. what happens when you lower the food bag over the edge and let go of the anchor bag? Determine the acceleration magnitude a of the two-bag system when you let go of the anchor bag?arrow_forward2. A thin Nichrome wire is used in an experiment to test Ohm's law using a power supply ranging from 0 to 12 V in steps of 2 V. Why isn't the graph of I vs V linear? 1. Nichrome wire does obey Ohm's law. Explain how that can that be true given the results abovearrow_forward1. The average KE and temperature in Kelvin of the molecules of a gas are related by the equation KE = 3/2 KT where k is the Boltzmann constant 1.38 x 10 m² kg s². The diagram shows the energy levels for a Hydrogen atom. Energy/eV 0.00 -1.51 3.39 13.58 Use this information to show that Hydrogen at room temperature will not emit light. 2. When hydrogen burns in oxygen 241.8 kJ of energy are released per mole. Show that this reaction can produce light.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning
Classical Dynamics of Particles and SystemsPhysicsISBN:9780534408961Author:Stephen T. Thornton, Jerry B. MarionPublisher:Cengage Learning Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill
Glencoe Physics: Principles and Problems, Student...PhysicsISBN:9780078807213Author:Paul W. ZitzewitzPublisher:Glencoe/McGraw-Hill College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University

Classical Dynamics of Particles and Systems
Physics
ISBN:9780534408961
Author:Stephen T. Thornton, Jerry B. Marion
Publisher:Cengage Learning

Glencoe Physics: Principles and Problems, Student...
Physics
ISBN:9780078807213
Author:Paul W. Zitzewitz
Publisher:Glencoe/McGraw-Hill

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning
Elastic and Inelastic Collisions; Author: Professor Dave Explains;https://www.youtube.com/watch?v=M2xnGcaaAi4;License: Standard YouTube License, CC-BY