
EBK ORGANIC CHEMISTRY
7th Edition
ISBN: 9780133556186
Author: Bruice
Publisher: VST
expand_more
expand_more
format_list_bulleted
Question
Chapter 9, Problem 1P
Interpretation Introduction
Interpretation:
The structure of DDE is to be drawn.
Concept Introduction:
Compounds in which one or more hydrogen atoms are replaced by halogen atoms are called
These alkyl halides usually undergo substitution or addition reactions.
The electronegative atom is replaced by another atoms or group are referred as substitution reactions.
The electronegative atom is eliminated along with a hydrogen from adjacent carbon is called elimination reactions.
Expert Solution & Answer
Answer to Problem 1P
The structure of DDE is,
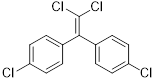
Explanation of Solution
DDE (dichlorodiphenyldichloroethylene) is a chemical compound forms as a result of loss of hydrogen chloride from DDT (dichlorodiphenyltrichloroethane).
The general reaction can be represented as:
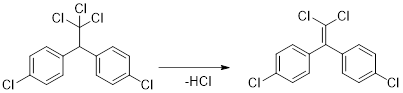
Conclusion
The structure of DDE was drawn.
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
Assign the functional group bands on the IR spectra.
Find the pH of a 0.120 M solution of HNO2.
Find the pH ignoring activity effects (i.e., the normal way).
Find the pH in a solution of 0.050 M NaCl, including activity
Please help me answer these three questions. Required info should be in data table.
Chapter 9 Solutions
EBK ORGANIC CHEMISTRY
Ch. 9.1 - Prob. 3PCh. 9.1 - Does increasing the energy barrier for an SN2...Ch. 9.1 - Rank the following alkyl bromides from most...Ch. 9.2 - Prob. 8PCh. 9.2 - Prob. 9PCh. 9.2 - Prob. 10PCh. 9.2 - Prob. 11PCh. 9.2 - Which substitution reaction lakes place more...Ch. 9.2 - Prob. 14PCh. 9.2 - Prob. 16P
Ch. 9.3 - Prob. 17PCh. 9.4 - Prob. 18PCh. 9.5 - Prob. 19PCh. 9.5 - Prob. 20PCh. 9.5 - Prob. 21PCh. 9.5 - Prob. 22PCh. 9.6 - Prob. 23PCh. 9.6 - Prob. 24PCh. 9.6 - Which of the following reactions take place more...Ch. 9.7 - Prob. 26PCh. 9.7 - Prob. 27PCh. 9.7 - Prob. 28PCh. 9.7 - Prob. 30PCh. 9.7 - Under which of the following reaction conditions...Ch. 9.8 - After a proton is removed from the OH group, which...Ch. 9.8 - Prob. 33PCh. 9.9 - Prob. 34PCh. 9 - Prob. 1PCh. 9 - Methoxychlor is an insecticide that was intended...Ch. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Prob. 37PCh. 9 - Prob. 38PCh. 9 - Prob. 39PCh. 9 - Prob. 40PCh. 9 - Starting with cyclohexene, how can the following...Ch. 9 - Prob. 42PCh. 9 - The pKa of acetic acid in water is 4.76. What...Ch. 9 - Prob. 44PCh. 9 - Prob. 45PCh. 9 - Prob. 46PCh. 9 - Prob. 47PCh. 9 - Prob. 48PCh. 9 - Prob. 49PCh. 9 - Prob. 50PCh. 9 - Prob. 51PCh. 9 - tert-Butyl chloride undergoes solvolysis in both...Ch. 9 - Prob. 53PCh. 9 - Prob. 54PCh. 9 - In which solventethanol or diethyl etherwould the...Ch. 9 - Prob. 56PCh. 9 - Two bromoethers are obtained from the reaction of...Ch. 9 - Prob. 58PCh. 9 - Prob. 59PCh. 9 - Prob. 60PCh. 9 - Propose a mechanism for the following reaction:Ch. 9 - Prob. 62PCh. 9 - Prob. 63PCh. 9 - Prob. 64PCh. 9 - Prob. 65PCh. 9 - When equivalent amounts of methyl bromide nod...Ch. 9 - Prob. 67PCh. 9 - The reaction of chloromethane with hydroxide ion...
Knowledge Booster
Similar questions
- Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each stereogenic center. Omit any byproducts. Bri CH3CH2O- (conc.) Draw the major organic product or products.arrow_forwardTartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH. How many mL of NaOH are needed to reach the first equivalence point? How many mL of NaOH are needed to reach the second equivalence point?arrow_forwardIncluding activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forward
- Order the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forwardCan I please get all final concentrations please!arrow_forward
- State the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forwardDo not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction. For the decomposition reaction of N2O5(g): 2 N2O5(g) · 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 -> NO2 + NO3_(K1) NO2 + NO3 →> N2O5 (k-1) → NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Give the expression for the acceptable rate. (A). d[N₂O] dt = -1 2k,k₂[N205] k₁+k₂ d[N₂O5] (B). dt =-k₁[N₂O₂] + k₁[NO2][NO3] - k₂[NO2]³ (C). d[N₂O] dt =-k₁[N₂O] + k₁[N205] - K3 [NO] [N205] (D). d[N2O5] =-k₁[NO] - K3[NO] [N₂05] dtarrow_forwardA 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 20.0 mL of the base solution, what is the pH of the resulting solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax


Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning