
Concept explainers
(a)
Interpretation: The total number of hydrogen atoms should be identified in ethane molecule.
Concept Introduction: The general formula of a hydrocarbon is 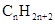 For hydrocarbons with 1 double bond the general formula is
For hydrocarbons with 1 double bond the general formula is 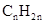 Similarly, for hydrocarbons with 1 triple bond the formula is
Similarly, for hydrocarbons with 1 triple bond the formula is 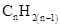 A hydrocarbon chain can be in cyclic or ring form, for that the general formula is
A hydrocarbon chain can be in cyclic or ring form, for that the general formula is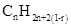 Here, r represents the number of rings.
Here, r represents the number of rings.
(b)
Interpretation: The total number of hydrogen atoms should be identified in 1-butyne
molecule.
Concept Introduction: The general formula of a hydrocarbon is 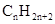 For hydrocarbons with 1 double bond the general formula is
For hydrocarbons with 1 double bond the general formula is 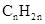 Similarly, for hydrocarbons with 1 triple bond the formula is
Similarly, for hydrocarbons with 1 triple bond the formula is 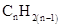 A hydrocarbon chain can be in cyclic or ring form, for that the general formula is
A hydrocarbon chain can be in cyclic or ring form, for that the general formula is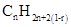 Here, r represents the number of rings.
Here, r represents the number of rings.
(c)
Interpretation: The total number of hydrogen atoms should be identified in propene
molecule.
Concept Introduction: The general formula of a hydrocarbon is 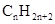 For hydrocarbons with 1 double bond the general formula is
For hydrocarbons with 1 double bond the general formula is 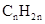 Similarly, for hydrocarbons with 1 triple bond the formula is
Similarly, for hydrocarbons with 1 triple bond the formula is 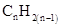 A hydrocarbon chain can be in cyclic or ring form, for that the general formula is
A hydrocarbon chain can be in cyclic or ring form, for that the general formula is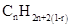 Here, r represents the number of rings.
Here, r represents the number of rings.
(d)
Interpretation: The total number of hydrogen atoms should be identified in cyclooctane
molecule.
Concept Introduction: The general formula of a hydrocarbon is 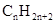 For hydrocarbons with 1 double bond the general formula is
For hydrocarbons with 1 double bond the general formula is 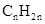 Similarly, for hydrocarbons with 1 triple bond the formula is
Similarly, for hydrocarbons with 1 triple bond the formula is 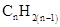 A hydrocarbon chain can be in cyclic or ring form, for that the general formula is
A hydrocarbon chain can be in cyclic or ring form, for that the general formula is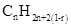 Here, r represents the number of rings.
Here, r represents the number of rings.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Chemistry For Changing Times (14th Edition)
- Propose a synthesis pathway for the following transformations. b) c) d)arrow_forwardThe rate coefficient of the gas-phase reaction 2 NO2 + O3 → N2O5 + O2 is 2.0x104 mol–1 dm3 s–1 at 300 K. Indicate whether the order of the reaction is 0, 1, or 2.arrow_forward8. Draw all the resonance forms for each of the following molecules or ions, and indicate the major contributor in each case, or if they are equivalent. (4.5 pts) (a) PH2 سمةarrow_forward
- 3. Assign absolute configuration (Rors) to each chirality center. a. H Nitz C. он b. 0 H-C. C H 7 C. ་-4 917-417 refs H 1つ ८ ડુ d. Но f. -2- 01 Ho -OH 2HNarrow_forwardHow many signals do you expect in the H NMR spectrum for this molecule? Br Br Write the answer below. Also, in each of the drawing areas below is a copy of the molecule, with Hs shown. In each copy, one of the H atoms is colored red. Highlight in red all other H atoms that would contribute to the same signal as the H already highlighted red. Note for advanced students: In this question, any multiplet is counted as one signal. Number of signals in the 'H NMR spectrum. For the molecule in the top drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in top molecule For the molecule in the bottom drawing area, highlight in red any other H atoms that will contribute to the same signal as the H atom already highlighted red. If no other H atoms will contribute, check the box at right. No additional Hs to color in bottom moleculearrow_forwardIn the drawing area below, draw the major products of this organic reaction: 1. NaOH ? 2. CH3Br If there are no major products, because nothing much will happen to the reactant under these reaction conditions, check the box under the drawing area instead. No reaction. Click and drag to start drawing a structure. ☐ : A คarrow_forward
- Predict the major products of the following organic reaction: NC Δ ? Some important Notes: • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to draw bonds carefully to show important geometric relationships between substituents. Note: if your answer contains a complicated ring structure, you must use one of the molecular fragment stamps (available in the menu at right) to enter the ring structure. You can add any substituents using the pencil tool in the usual way. Click and drag to start drawing a structure. Х аarrow_forwardPredict the major products of this organic reaction. Be sure you use dash and wedge bonds to show stereochemistry where it's important. + ☑ OH 1. TsCl, py .... 文 P 2. t-BuO K Click and drag to start drawing a structure.arrow_forwardConsider this organic reaction: ( Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. Click and drag to start drawing a structure. Х : а ค 1arrow_forward
- In the drawing area below, draw the major products of this organic reaction: If there are no major products, because nothing much will happen to the reactant under these reaction conditions, check the box under the drawing area instead. 1. NaH 2. CH3Br ? Click and drag to start drawing a structure. No reaction. : ☐ Narrow_forward+ Predict the major product of the following reaction. : ☐ + ☑ ค OH H₂SO4 Click and drag to start drawing a structure.arrow_forwardConsider this organic reaction: ... OH CI Draw the major products of the reaction in the drawing area below. If there won't be any major products, because this reaction won't happen at a significant rate, check the box under the drawing area instead. ☐ No Reaction. Click and drag to start drawing a structure. : аarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





