
a)
The thermal efficiency of the cycle using the constant specific heats at room temperature.
a)
Explanation of Solution
Given:
Compression ratio
Temperature of air at state 1
Temperature of air at state 3
Pressure of air at state 1
Calculation:
Draw the
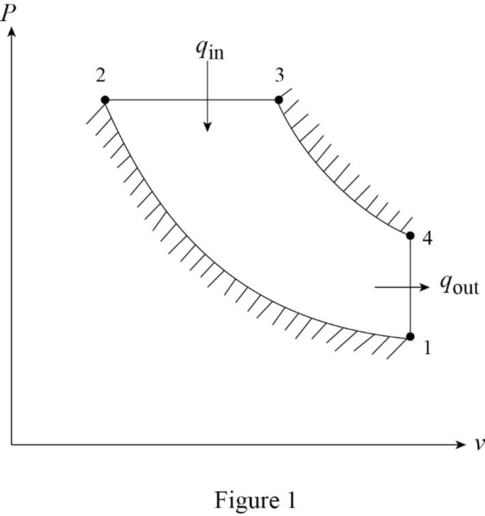
Refer Table A-2E, “Ideal-gas specific heats of various common gases”, obtain the following properties of the air.
Calculate the temperature at state 2
Calculate the ratio between the volumes at state 3 and state 2
Calculate the temperature at state 4
Calculate the net specific work produced by the cycle
Calculate the thermal efficiency of the cycle
Thus, the thermal efficiency of the cycle using the constant specific heats at room temperature is
b)
The thermal efficiency of the cycle using the variable specific heats.
b)
Explanation of Solution
Calculation:
Refer table A-21E, “Ideal gas properties of the air”, obtain the specific internal energy and relative specific volume of air at the temperature of
Calculate the relative specific volume at state 2
Refer table A-21E, “Ideal gas properties of the air”, obtain the specific enthalpy and temperature of air at the relative pressure of
Refer table A-21E, “Ideal gas properties of the air”, obtain the specific enthalpy and relative specific volume of air at the temperature of
Calculate the ratio between the specific volumes at state 3 and state 2
Calculate the relative specific volume at state 4
Refer table A-21E, “Ideal gas properties of the air”, obtain the specific internal energy of air at the relative specific volume of
Calculate the thermal efficiency of the cycle
Thus, the thermal efficiency of the cycle using the variable specific heats is
Want to see more full solutions like this?
Chapter 9 Solutions
Fundamentals Of Thermal-fluid Sciences In Si Units
- PROBLEM 3.46 The solid cylindrical rod BC of length L = 600 mm is attached to the rigid lever AB of length a = 380 mm and to the support at C. When a 500 N force P is applied at A, design specifications require that the displacement of A not exceed 25 mm when a 500 N force P is applied at A For the material indicated determine the required diameter of the rod. Aluminium: Tall = 65 MPa, G = 27 GPa. Aarrow_forwardFind the equivalent mass of the rocker arm assembly with respect to the x coordinate. k₁ mi m2 k₁arrow_forward2. Figure below shows a U-tube manometer open at both ends and containing a column of liquid mercury of length l and specific weight y. Considering a small displacement x of the manometer meniscus from its equilibrium position (or datum), determine the equivalent spring constant associated with the restoring force. Datum Area, Aarrow_forward
- 1. The consequences of a head-on collision of two automobiles can be studied by considering the impact of the automobile on a barrier, as shown in figure below. Construct a mathematical model (i.e., draw the diagram) by considering the masses of the automobile body, engine, transmission, and suspension and the elasticity of the bumpers, radiator, sheet metal body, driveline, and engine mounts.arrow_forward3.) 15.40 – Collar B moves up at constant velocity vB = 1.5 m/s. Rod AB has length = 1.2 m. The incline is at angle = 25°. Compute an expression for the angular velocity of rod AB, ė and the velocity of end A of the rod (✓✓) as a function of v₂,1,0,0. Then compute numerical answers for ȧ & y_ with 0 = 50°.arrow_forward2.) 15.12 The assembly shown consists of the straight rod ABC which passes through and is welded to the grectangular plate DEFH. The assembly rotates about the axis AC with a constant angular velocity of 9 rad/s. Knowing that the motion when viewed from C is counterclockwise, determine the velocity and acceleration of corner F.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





