
Chemistry Atoms First2e
2nd Edition
ISBN: 9781947172647
Author: OpenStax
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 9, Problem 11E
A piece of unknown solid substance weighs 437.2 g. and requires 8460 J to increase its temperature from 19.3°C to 68.9°C.
(a) What is the specific heat of the substance?
(b) If it is one of the substances found in Table 9.1, what is its likely identity?
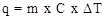
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
In the electrode Pt, H2(1 atm) | H+(a=1), if the electrode balance potential is -0.118 V and the interface potential difference is +5 mV. The current voltage will be 0.005 - (-0.118) = 0.123 V ¿Correcto?
In the electrode Pt, H2(1 atm) | H+(a=1) at 298K is 0.79 mA cm-2. If the balance potential of the electrode is -0.118 V and the potential difference of the interface is +5 mV. Determine its potential.
In one electrode: Pt, H2(1 atm) | H+(a=1), the interchange current density at 298K is 0.79 mA·cm-2. If the voltage difference of the interface is +5 mV. What will be the correct intensity at pH = 2?. Maximum transfer voltage and beta = 0.5.
Chapter 9 Solutions
Chemistry Atoms First2e
Ch. 9 - A burning match and a bonfire may have the same...Ch. 9 - Prepare a table identifying several energy...Ch. 9 - Explain the difference between heat capacity and...Ch. 9 - Calculate the heat capacity, in joules and in...Ch. 9 - Calculate the heat capacity, in joules and in...Ch. 9 - How much heat, in joules and in calories, must be...Ch. 9 - How much heat, in joules and in calories, is...Ch. 9 - How much would the temperature of 275 g of water...Ch. 9 - If 14.5 kJ of heat were added to 485 g of liquid...Ch. 9 - A piece of unknown substance weighs 44.7 g and...
Ch. 9 - A piece of unknown solid substance weighs 437.2 g....Ch. 9 - An aluminum kettle weighs 1.05 kg. (a) What is the...Ch. 9 - Most people find waterbeds uncomfortable unless...Ch. 9 - A 500-mL bottle of water at room temperature and a...Ch. 9 - Would the amount of heat measured for the reaction...Ch. 9 - Would the amount of heat absorbed by the...Ch. 9 - Would the amount of heat absorbed by the...Ch. 9 - How many milliliters of water at 23 C with a...Ch. 9 - How much will the temperature of a cup (180 g) of...Ch. 9 - A 45-g aluminum spoon (specific heat 0.88 J/g C)...Ch. 9 - The temperature of the cooling water as it leaves...Ch. 9 - A 70.0-g piece of metal at 80.0 C is placed in 100...Ch. 9 - If a reaction produces 1.506 kJ of heat, which is...Ch. 9 - A 0.500-g sample of KCI is added to 50.0 g of...Ch. 9 - Dissolving 3.0 g of CaCl2(s) in 150.0 g of water...Ch. 9 - When 50.0 g of 0.200 M NaCl(aq) at 24.1 C is added...Ch. 9 - The addition of 3.15 g of Ba(OH)28H2O to a...Ch. 9 - The reaction of 50 mL of acid and 50 mL of base...Ch. 9 - If the 3.21 g of NH4NO3 in Example 9.6 were...Ch. 9 - When 1.0 g of fructose, C6H12O6(s), a sugar...Ch. 9 - When a 0.740-g sample of trinitrotoluene (TNT),...Ch. 9 - One method of generating electricity is by burning...Ch. 9 - The amount of fat recommended for someone with a...Ch. 9 - A teaspoon of the carbohydrate sucrose (common...Ch. 9 - What is the maximum mass of carbohydrate in a 6-oz...Ch. 9 - A pint of premium ice cream can contain 1100...Ch. 9 - A serving of a breakfast cereal contains 3 g of...Ch. 9 - Which is the least expensive source of energy in...Ch. 9 - Explain how the heat measured in Example 9.5...Ch. 9 - Using the data in the check your learning section...Ch. 9 - Calculate the enthalpy of solution( H for the...Ch. 9 - Calculate H for the reaction described by the...Ch. 9 - Calculate the enthalpy of solution ( H for the...Ch. 9 - Although the gas used in an oxyacetylene torch...Ch. 9 - How much heat is produced by burning 4.00 moles of...Ch. 9 - How much heat is produced by combustion of 125 g...Ch. 9 - How many moles of isooctane must be burned to...Ch. 9 - What mass of carbon monoxide must be burned to...Ch. 9 - When 2.50 g of methane burns in oxygen, 125 kJ of...Ch. 9 - How much heat is produced when loo mL of 0.250 M...Ch. 9 - A sample of 0.562 g of carbon is burned in oxygen...Ch. 9 - Before the introduction of chlorofluorocarbons,...Ch. 9 - Homes may be heated by pumping hot water through...Ch. 9 - Which of the enthalpies of combustion in Table 9.2...Ch. 9 - Does the standard enthalpy of formation of H2O(g)...Ch. 9 - Joseph Priestly prepared oxygen in 1774 by heating...Ch. 9 - How many kilojoules of heat will be released when...Ch. 9 - How many kilojoules of heat will be released when...Ch. 9 - The following sequence of reactions occurs in the...Ch. 9 - Both graphite and diamond burn....Ch. 9 - From the molar heats of formation in Appendix G,...Ch. 9 - Which produces more heat?...Ch. 9 - Calculate H for the process Sb(s)+52Cl2(g)SbCl5(s)...Ch. 9 - Calculate H for the process...Ch. 9 - Calculate H for the process Hg2Cl2(s)2Hg(l)+Cl2(g)...Ch. 9 - Calculate H for the process Co3O4(s)3Co(s)+202(g)...Ch. 9 - Calculate the standard molar enthalpy of formation...Ch. 9 - Using the data in Appendix G, calculate the...Ch. 9 - Using the data in Appendix G, calculate the...Ch. 9 - The following reactions can be used to prepare...Ch. 9 - The decomposition of hydrogen peroxide, H2O2, has...Ch. 9 - Calculate the enthalpy of combustion of propane,...Ch. 9 - Calculate the enthalpy of combustion of butane,...Ch. 9 - Both propane and butane are used as gaseous fuels....Ch. 9 - The white pigment TiO2 is prepared by the reaction...Ch. 9 - Water gas, a mixture of H2 and CO2 is an important...Ch. 9 - In the early days of automobiles, illumination at...Ch. 9 - From the data in Table 9.2, determine which of the...Ch. 9 - The enthalpy of combustion of hard coal averages...Ch. 9 - Ethanol, C2H5OH, is used as a fuel for motor...Ch. 9 - Among the substances that react with oxygen and...Ch. 9 - How much heat is produced when 1.25 g of chromium...Ch. 9 - Ethylene, C2H2, a byproduct from the fractional...Ch. 9 - The oxidation of the sugar glucose, C6H12O6, is...Ch. 9 - Propane, C3H8, is a hydrocarbon that is commonly...Ch. 9 - During a recent winter month in Sheboygan,...Ch. 9 - Which bond in each of the following pairs of bonds...Ch. 9 - Using the bond energies in Table 9.3, determine...Ch. 9 - Using the bond energies in Table 9.3, determine...Ch. 9 - Draw a curve that describes the energy of a system...Ch. 9 - Explain why bonds occur at specific average bond...Ch. 9 - When a molecule can form two different structures,...Ch. 9 - How does the bond energy of HCl(g) differ from the...Ch. 9 - Using the standard enthalpy of formation data in...Ch. 9 - Using the standard enthalpy of formation data in...Ch. 9 - Using the standard enthalpy of formation data in...Ch. 9 - Using the standard enthalpy of formation data in...Ch. 9 - Complete the following Lewis structure by adding...Ch. 9 - Use the bond energy to calculate an approximate...Ch. 9 - Use principles of atomic structure to answer each...Ch. 9 - The lattice energy of LiF is 1023 kJ/mol, and the...Ch. 9 - For which of the following substances is the least...Ch. 9 - The reaction of a metal, M, with a halogen, X2,...Ch. 9 - The lattice energy of LiF is 1023 kJ/mol, and the...Ch. 9 - Which compound in each of the following pairs has...Ch. 9 - Which compound in each of the following pairs has...Ch. 9 - Which of the following compounds requires the most...Ch. 9 - Which of the following compounds requires the most...Ch. 9 - The lattice energy of KF is 794 kJ/mol, and the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In a Pt electrode, H2(1 atm) | H+(a=1), the interchange current density of an electrode is 0.79 mA cm-2. ¿Qué corriente flow across the electrode of área 5 cm2 when the difference in potential of the interface is +5 mV?.arrow_forwardIf the current voltage is n = 0.14 V, indicate which of the 2 voltage formulas of the ley of Tafel must be applied i a a) == exp (1-B). xp[(1 - ß³): Fn Fn a b) == exp B RT RTarrow_forwardIf the current voltage is n = 0.14 V. Indicate which of the 2 formulas must be applied a) = a T = i exp[(1 - p) F Fn Fn b) i==exp B RTarrow_forward
- Topic: Photochemistry and Photophysics of Supramoleculesarrow_forwardTwo cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 Carrow_forwardWith the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY